Le Infezioni in Medicina, n. 4, 445-447, 2025
doi: 10.53854/liim-3304-9
CASE REPORTS
Deltoid Administration of LAI-ART: Pharmacokinetic Implications When the Gluteal Site Is Not Feasible
Maria Vittoria Cossu1, Antonio D’Avolio2, Anna Lisa Ridolfo1, Davide Moschese1, Andrea Gori 1, Dario Cattaneo3,4, Cristina Gervasoni1
1 Department of Infectious Diseases, ASST Fatebenefratelli Sacco University Hospital, Milan, Italy;
2 Laboratory of Clinical Pharmacology and Pharmacogenetics, Amedeo di Savoia Hospital, Department of Medical Sciences, University of Turin, Turin, Italy;
3 Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy;
4 Clinical Analysis Laboratory, IRCCS Humanitas Research Hospital, Rozzano, Italy.
Article received 28 July 2025 and accepted 14 October 2025
Corresponding author
Cristina Gervasoni
E-mail: cristina.gervasoni@asst-fbf-sacco.it
SUMMARY
This case report describes the use of long-acting injectable (LAI) antiretrovirals administered via an alternative injection site in an individual with HIV who was unable to receive intramuscular gluteal injections. Specifically, it focuses on intensive pharmacokinetic monitoring following deltoid administration of LAI rilpivirine and cabotegravir in a 29-year-old transgender woman with progressive multifocal leukoencephalopathy and swallowing difficulties. Gluteal injection was contraindicated due to prior silicone injections in that region. Peak plasma concentrations were reached on day 3 post-injection for both drugs, with cabotegravir peaking at 1278 ng/mL and rilpivirine at 30 ng/mL. Rilpivirine plasma levels declined more rapidly than cabotegravir levels. By day 10, the patient’s clinical condition had deteriorated, leading to hospice care and subsequent death. Rilpivirine exposure after deltoid injection appeared lower than that typically reported after gluteal or thigh administration, although intracellular drug concentrations may differ from plasma levels, potentially mitigating clinical concerns.
Keywords: Long-acting injectable antiretrovirals, Cabotegravir, Rilpivirine, deltoid administration, pharmacokinetics.
INTRODUCTION
Providing long-acting injectable (LAI) antiretrovirals at alternative anatomical sites represents a critical option for people with HIV (PWH) who have contraindications to intramuscular gluteal administration. To date, only limited data are available on the pharmacokinetics (PK) of cabotegravir and rilpivirine administered from alternative injection sites [1, 2]. Han et al. evaluated the lateral thigh as an alternative injection site in fifteen healthy adults who received oral dosing followed by a single intramuscular injection into the thigh [1]. Drug levels remained well above therapeutic targets for at least 8 weeks. Injection-site reactions were common but mild to moderate. The thigh site showed pharmacokinetics and tolerability profiles comparable to those observed after gluteal injections, supporting its use as a potential alternative. Subsequently, we reported two cases of transgender women who required deltoid administration of LAI due to prior gender-affirming procedures involving the gluteal and thigh regions with injected silicon. Both patients maintained therapeutic trough concentrations of rilpivirine and cabotegravir [2].
We report here the results of intensive PK monitoring over the first 10 days following separate administrations of LAI rilpivirine and cabotegravir into the contralateral deltoid muscles of a patient with severe neurological decline and swallowing impairment, who had previously undergone silicone injections in the gluteal region.
CASE REPORT
A 29-year-old transgender woman with a recent diagnosis of HIV infection was admitted to our hospital for progressive cognitive deterioration and both cutaneous and visceral Kaposi’s sarcoma. Her baseline CD4 count and plasma HIV RNA level were 207 cells/µL (18.2%) and 1,530,000 copies/mL, respectively. A diagnosis of progressive multifocal leukoencephalopathy (PML) was made following detection of JC virus (JCV) DNA in cerebrospinal fluid.
Initial antiretroviral therapy (ART) included tenofovir alafenamide/emtricitabine/bictegravir. Due to rapid neurological deterioration and loss of swallowing function, compounded by repeated dislodgement of the nasogastric feeding tube, ART was switched to LAI regimen, despite the absence of complete virological suppression (HIV RNA: 292 copies/mL). Given a history of extensive silicone injections in the gluteal and thigh regions (performed in 2017), it was decided to administer the LAI cabotegravir and rilpivirine off label in the deltoid muscles. At the start of LAI treatment, the patient was receiving only trimethoprim-sulfamethoxazole, a proton pump inhibitor, low-molecular-weight heparin, and parenteral nutrition.
Intensive PK monitoring was conducted during the first 10-days post-injection to quantify drug exposure from this alternative route. Plasma concentrations of rilpivirine and cabotegravir were quantified using an ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS), as previously described [3]. The assay demonstrated linearity over the ranges of 10 to 2500 ng/mL for rilpivirine and 40 to 10,000 ng/mL for cabotegravir. Plasma concentrations of cabotegravir and rilpivirine were measured daily at the same time. As shown in Figure 1, both drugs reached peak concentrations on day 3 after post-injection (Cmax 1278 for cabotegravir and 30 ng/mL for rilpivirine). Thereafter, plasma levels declined gradually, with rilpivirine exhibiting a more rapid decrease than cabotegravir.
Unfortunately, the patient’s clinical condition continued to deteriorate, and she was transferred to hospice care on day 12, where she subsequently passed away. At the last available evaluation (day 10 post-injection) HIV RNA was 172 copies/mL, and plasma concentrations of cabotegravir and rilpivirine were 1190 and 12 ng/mL, respectively.
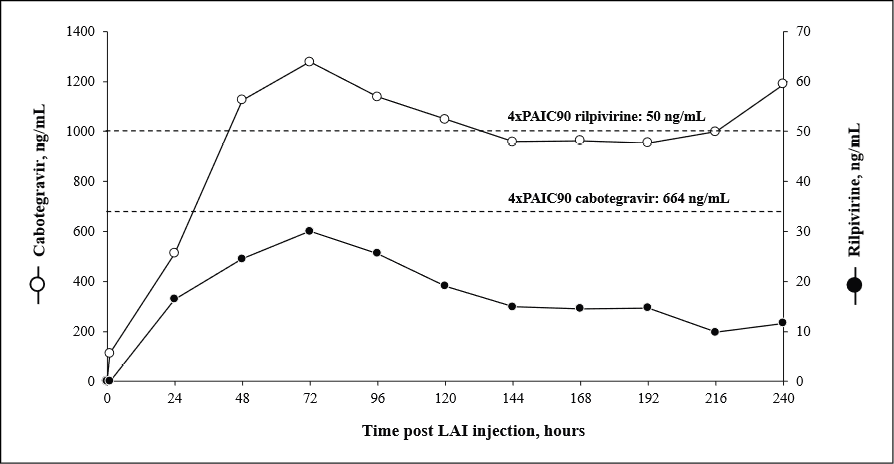
Figure 1 - Time-course of cabotegravir and rilpivirine plasma concentrations measured in the first 10 days after intramuscular deltoid injection. Dotted lines represented the 4xPA-IC90 (664 ng/mL for cabotegravir and 50 ng/mL for rilpivirine).
DISCUSSION
We have recently reported two cases of transgender women requiring a switch to deltoid administration of LAI ART due to prior gender-affirming surgery both of whom maintained therapeutic trough concentrations of rilpivirine and cabotegravir [2]. Both patients started LAI antiretrovirals with a controlled HIV viremia. In both cases, trough levels at week 24 post-switch remained well above the therapeutic targets (664 ng/mL for cabotegravir and 50 ng/mL for rilpivir ine, corresponding to 4xPA-IC90). These findings contrast with the current case in which rilpivirine concentrations remained below the therapeutic threshold throughout the 10-day monitoring period, while cabotegravir levels exceeded the target from day 3 onward.
These discrepancies may, at least in part, be attributed to differences in sampling timing or prior drug exposure. In addition, they might be influenced by patient-related factors, such as cachexia although no data are available to support this. In the previously reported cases, patients had been on ART for over four years prior to the LAI switch and PK monitoring was performed under steady-state conditions.
Conversely, in the present case, both agents were administered via the deltoid route for the first time, and PK data reflect early exposure phases in a LAI-naïve individual. Suboptimal rilpivirine exposure in the early phases following gluteal or thigh injections has also been described in both healthy volunteers and PWH [4,5]. However, deltoid administration in this patient was associated with even lower Cmax values (30 vs. 80-100 ng/mL) and shorter Tmax (3 vs. 5–7 days) compared with previous reports. Recent data by Ferrara et al demonstrated that the intracellular/plasma concentration ratios differ markedly between the two agents – approximately 15% for cabotegravir and 800% for rilpivirine [6]. These findings suggest that plasma concentrations of rilpivirine may not accurately reflect intracellular drug exposure, potentially limiting the clinical relevance of the observed plasma underexposure.
Important limitations of this case report should be acknowledged. Pharmacokinetic monitoring was limited to a 10-day period, which is insufficient to draw definitive conclusions about a LAI antiretrovirals administration via the deltoid route. Furthermore, the patient’s rapid clinical decline and short follow-up precluded meaningful assessment of therapeutic durability or clinical relevance. Finally, the potential confounding effects of advanced HIV infection and PML on the observed modest viral load decline cannot be excluded.
In conclusion, this case highlights the complexity of managing HIV infection when oral administration is not feasible and underscores the potential of LAI ART regimens as a viable alternative, at least in PWH with undetectable HIV-RNA. However, based on our experience, we advise caution when considering the deltoid route for initiating LAI ART in treatment-naïve individuals. This approach may be more appropriate for patients who are already receiving LAI therapy and require a change in injection site.
Conflict of interest
All the Authors declare no conflict of interest.
Funding
This study was carried out as part of our routine work.
REFERENCES
[1] Han K, Gevorkyan H, Sadik Shaik J, et al. Pharmacokinetics and tolerability of cabotegravir and rilpivirine long-acting intramuscular injections to the vastus lateralis (lateral thigh) muscles of healthy adult participants. Antimicrob Agents Chemother. 2024; 68(1): e0078123.
[2] Cossu MV, D’Avolio A, Gervasoni C, Giacomelli A, Cattaneo D, Moschese D. Switching to deltoid intramuscular injections maintains therapeutic trough concentrations of rilpivirine and cabotegravir in people with HIV. Antimicrob Agents Chemother. 2024; 68(5): e0017524.
[3] Cossu MV, Cattaneo D, Moschese D, et al. Rilpivirine and cabotegravir trough concentrations in people with HIV on long-term treatment with long-acting injectable antiretrovirals. J Antimicrob Chemother. 2024; 79(5): 1126-1132.
[4] Verloes R, Deleu S, Niemeijer N, Crauwels H, Meyvisch P, Williams P. Safety, tolerability and pharmacokinetics of rilpivirine following administration of a long-acting formulation in healthy volunteers. HIV Med. 2015; 16(8): 477-484.
[5] Neyens M, Crauwels HM, Perez-Ruixo JJ, Rossenu S. Population pharmacokinetics of the rilpivirine long-acting formulation after intramuscular dosing in healthy subjects and people living with HIV. J Antimicrob Chemother. 2021; 76(12): 3255-3262.
[6] Ferrara M, Maccario V, Barrera F, et al. Long acting cabotegravir and rilpivirine plasma and intracellular pharmacokinetics in the clinical setting. Sex Transmitted Infections. 2024; 100(Suppl. 1): A17.