Le Infezioni in Medicina, n. 2, 213-221, 2024
doi: 10.53854/liim-3202-9
ORIGINAL ARTICLES
Adherence to universal screening for group B Streptococcus in pregnancy and prevalence of colonised pregnancies in Caserta province, Italy
Salvatore Porzio1, Maurizio Bianchi2
1Dipartimento Materno-infantile, Casa di Cura San Michele, Maddaloni (Caserta), Italia;
2Department of Translational Medical Science, University of Naples Federico II, Naples, Italy
Article received 24 December 2023, accepted 12 April 2024
Corresponding author
Salvatore Porzio
E-mail: nidosanmichele@gmail.com
SummaRY
Group B Streptococcus (Streptococcus agalactiae; GBS) infection is a significant contributor to neonatal morbidity and mortality. In the early 1970s, the neonatal mortality rate for infants with invasive GBS disease was 55%. With the adoption of the first medical community guidelines to prevent GBS infection in the 1990s, the mortality rate decreased to approximately 5%. The main obstetric procedure for preventing vertical transmission of GBS infection involves universal screening of pregnant women using a vaginal-rectal swab (VRS) to identify those eligible for intrapartum antibiotic prophylaxis (IAP). The study analyzes the adherence of screening and the trend of GBS infection in pregnancy in the province of Caserta, Italy. Data were obtained from pregnant women who gave birth in a first level birthing center in 2022 from birth assistance certificate (CEDAP), obstetric and neonatal record. Postnatal evaluation collected through computer-assisted telephone interviews. 567 women delivered at our center during the study period. The average coverage of GBS testing in pregnancy was 99.2% (562), and the proportion of GBS colonised women was 12.6% (71) according with the national average, which is about 10-20%. The spread of positive cases appears to fluctuate among the various groups of pregnant women studied, indicating no significant statistical variance among presence of a partner, among women who have given birth multiple times, among Italian nationals, or across different ages, but a significant statistical excess is evident among mothers with less education. In 93% (66) of GBS carrier mothers, intrapartum antibiotic prophylaxis (IAP) was administered correctly, regardless of the type of delivery performed. Despite the successful integration of GBS screening, a significant gap remains between the ideal scenario and the actual implementation of IAP. At the three-month assessment, no child required hospitalization, consistent with the relatively low incidence of invasive GBS infection. Nevertheless, for those who are not eligible to VRS screening, such as preterm birth, or IAP, as in precipitous birth, the identification of biomarkers enabling early recognition of invasive GBS disease remains essential. Additionally, the emergence of vaccines administered during gestation, conferring passive immunity to newborns represents a promising possible new direction. Therefore, to ensure the practical application of GBS screening and actual IAP by healthcare providers, periodic audits and regular monitoring should be encouraged.
Keywords: Group B streptococcus (GBS), universal prenatal screening, vaginal-rectal swab, neonatal infection.
INTRODUCTION
Group B Streptococcus (GBS) is a significant cause of bacterial infections in newborns, causing both early-onset diseases (EOD) within the first 7 days of life and late-onset diseases (LOD) within 90 days of life [1]. Invasive GBS disease typically presents with nonspecific symptoms such as respiratory distress, which is the most common sign of EOD. In contrast, LOD forms often begin with fever, lethargy, and poor feeding [2]. In the 1970s, invasive GBS disease presented a significant worldwide challenge with a mortality rate around 55% [3, 4]. At present, the estimated GBS infection rate varies from 0.21 to 2.00 per 1,000 live births, with a case fatality rate (CFR) ranging from 4.7% to 18.9% among different countries. In 2017, the incidence rates for EOD exceeded those for LOD globally, with rates of 0.41 and 0.26 per 1,000 live births, respectively. The case fatality rates (CFRs) were 10% and 7% for EOD and LOD, respectively [5]. Approximately 80% of GBS neonatal sepsis is attributable to transmission during labor and delivery but only 1-2% of infants born to colonised mothers develop EOD [6, 7]. Following the implementation of guidelines and utilization of prenatal screening for GBS and IAP for all GBS carriers, an 80% decrease in early onset cases has been observed, and the mortality rate fell to around 5% [8, 9]. However, in cases where prenatal screening was not performed and risk factor assessment was conducted solely during labor, the EOD cases occurred either among eligible mothers who did not receive IAP or those who received insufficient IAP (45.4% and 71.4%, respectively) [6]. Despite satisfactory GBS screening implementation, there is still a substantial gap between optimal and actual IAP [10]. Therefore, educating expectant mothers about the importance of GBS screening and treatment is crucial. However, it is equally important for healthcare providers to adhere to guideline recommendations and ensure their practical application, and periodic audits and regular monitoring should be encouraged. The aim of the study is to assess adherence to universal GBS screening with VRS in a primary level birthing center. Secondary objectives are to evaluate the prevalence of GBS colonization in the same parturients, the incidence of GBS infection and the proper implementation of IAP.
PATIENTS AND METHODS
As recommended by the national guidelines for the assistance of physiological pregnancy, the GBS testing protocol provides for a vaginal-rectal swab to be performed between the 36th and 37th week. Two swabs (vaginal and rectal), are routinely performed by the clinicians before the digital examination and avoiding the use of the speculum when taking the vaginal sample. If there is a threat of preterm labor, or swab is not available a vaginal swab and a rectal swab are performed. The result of the vaginal-rectal swab is generally recorded in the personal obstetric guide of the pregnant woman and systematically transcribed in neonatal record. We considered data from birth assistance certificate (CEDAP), as primary information source, of all assisted pregnant women from January to December 2022 consisting in all 567 infants born in primary level birthing center Casa di Cura San Michele, Maddaloni - Caserta, Italy. For gathering additional information, we collected data from obstetric and neonatal records, as well as postnatal details through computer-assisted telephone interviews up to the age of three months, resulting in the construction of a computerized database. Access to medical records and authorization for interview was expressly authorized for this task. The study ended in March 2023. Data collection includes demographic information for pregnant women, including their citizenship, age, education, pregnancy record, clinical and laboratory information regarding the mother and the newborn, infectious disease history (e.g., TORCH, GBS) and therapeutic management (Table 1). During the computer assisted telephone interviews we collected the following data: clinical information regarding the newborn and history of hospitalizations for GBS sepsis (Table 2).
Table 1 - Characteristics of the cohort.

Table 2 - Follow-up questionnaire.
Statistical analysis
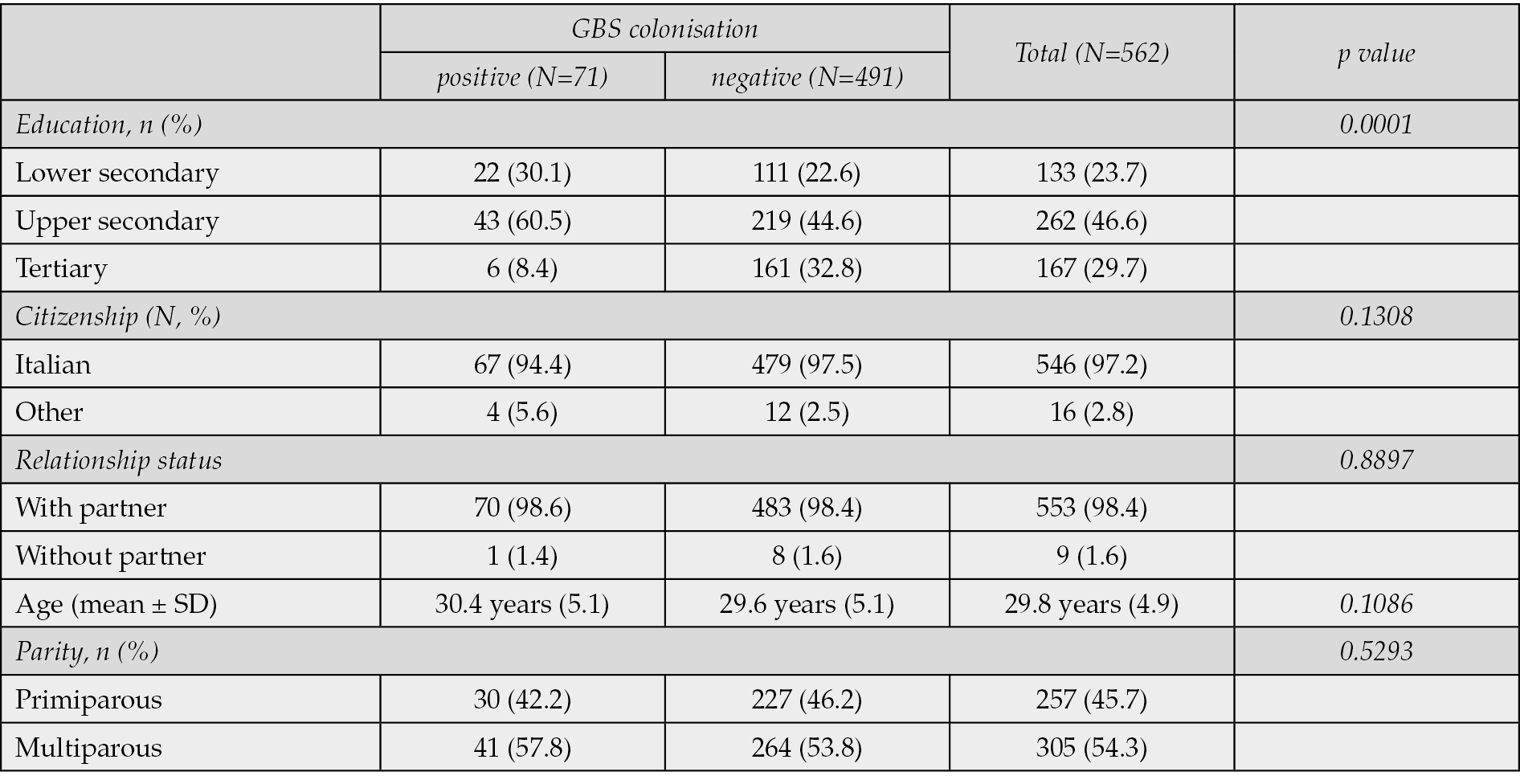
The prevalence was estimated in terms of frequencies, percentages and standard deviation (Tables 1, 3, 4). Then the factors that influence the prevalence of GBS carriage were explored. Statistical analysis was performed using the t-Student tests for quantitative variables and the chi-squared tests for qualitative variables. The level of statistical significance was set to p=0.05 (Table 3).
Table 3 - Sociodemographic characteristics of GBS positive mothers vs GBS negative mothers.
RESULTS
Five hundred and sixty-two of 567 pregnant women enrolled between January and December 2022 had been screened for GBS between 36-37 weeks of pregnancy. Screening coverage for GBS is therefore 99,2%. Only 5 pregnant women were not screened according to the protocol and VRS was carried out after delivery. Seventy-one pregnant women were positive for GBS corresponding to a prevalence rate of 12.6%. All collected data are reported in Table 1. In our study population, the mean gestational age at birth was 38 weeks and 6 days (±1.3 SD), with a mean birth weight of 3293g (± 3293 SD), and a 5-minute Apgar score of 9 (±0.4 SD). 24.3% of pregnant women reported low educational attainment (lower secondary). Multiparous women constituted 54.7% of the sample, and none of them reported a history of GBS infection in a previous newborn. The average age of participants was 29.8 (±4.9 SD) years and 46.4% of pregnancies ended with spontaneous delivery. In 5 (7%, N=71) cases (precipitated deliveries) no prophylaxis is documented or an IAP inadequate for timing and/or dosage, as recommended by American College of Obstetricians and Gynecologists (ACOG) [11]. In all observed cases, the reason was precipitous labor and temporal impossibility to administer prophylaxis. Furthermore, in our experience, prophylaxis was administered regardless of the indication for spontaneous vaginal delivery or cesarean section, despite cesarean delivery posing a low risk for vertical transmission of GBS according to ACOG. Ampicillin is the drug of choice for IAP. Ampicillin dosage, in case of premature rupture of the membranes, is initially 2 g by intravenous infusion, then 1 g every 4 hours until delivery. Alternatively, cefazolin, initially 2 g by intravenous infusion, followed by 1g every 8 hours until delivery. At the follow-up, of the 567 mothers contacted by telephone, 549 completed the survey 90 days after delivery (96.82%): among 71 GBS carrier mothers, 69 completed the survey (97.2%). Out of the 496 mothers negative for GBS screening, 480 completed the survey (96.7%). Neonatal EOD or LOD did not occur in any of the children born from both GBS-carrying and non-carrying mothers. The distribution of positive cases seems to vary across different categories of pregnant women examined, showing no statistically significant differences among presence of a partner, among multiparous women, among Italians and foreign women, or across age groups. However, there is a notable statistical excess observed solely among mothers with lower levels of education (Table 3).
DISCUSSION
With the implementation of universal screening via VRS for GBS during the 35th to 37th weeks of gestation and administration of intrapartum antibiotic prophylaxis when indicated, in adherence to the 1996 CDC guidelines, the occurrence of EOD has been significantly reduced [12]. Updated in 2019 by ACOG and by American Academy of Pediatrics (AAP), the guidelines now recommend screening during the 36th to 37th weeks of pregnancy [13]. In fact, GBS colonisation may be intermittent or transitory, resulting in a low positive predictive value of a culture test performed more than five weeks before delivery and limited clinical usefulness [14]. In addition, false negatives may arise due to the culture’s limited sensitivity or acquisition of GBS between screening and delivery. The noteworthy number of cases of EOD and LOD that present in infants born to GBS-negative women subjected to screening, poses concerns regarding identification of GBS colonisation [8]. This issue may be impacted by factors such as timing, methods, and sample transportation and processing. The ongoing advancement of diagnostic techniques facilitates the more accurate and punctual identification of GBS. Real-time PCR magnifies genes that are species-specific and conserved, like the cfb gene, with elevated sensitivity and reduced time to outcome [15]. Carrillo-Ávila et al. conducted a study comparing the gold standard culture method to PCR, which demonstrated the high sensitivity (95.5%) and specificity (99.13%) of the molecular method [16]. False negatives in real-time PCR may result from a low bacterial count, leading to low DNA concentration in samples. A modified DNA extraction procedure not adhering to CDC recommendations could be another possible reason. Nevertheless, the inability to carry out antibiotic susceptibility testing is a significant restriction of these methods [17]. Although culture testing should remain the primary and regular technique due to its specificity and cost-effectiveness, as well as its ability to conduct antibiotic sensitivity tests, using PCR techniques would be advantageous for negative culture samples or urgent detection. Furthermore, colonisation of GBS during pregnancy increases the likelihood of spontaneous abortion, preterm labour, premature rupture of fetal membranes, and low birth weight in neonates [18]. From the literature review, it remains evident that adherence to universal GBS screening varies considerably. A study conducted between July and December 2013, involving 468 women and 475 live births admitted to the Obstetric and Gynecology Unit of Cardarelli Hospital (Campobasso, Italy), revealed that only 241 pregnant women (51.5%) underwent appropriate vaginal-rectal screening for GBS. Additionally, vaginal cultures alone were obtained from 77 pregnant women (16.4%) [19]. A study conducted in Trento, Italy, on 21,209 live births between 2015 and 2019 showed an adherence rate to screening with vaginal-rectal swab VRS of 91.8%. In Piemonte, northern Italy, 12.6% of pregnant women did not performed GBS swab testing or did not have a swab available at the time of delivery [20]. Numerous risk factors are reported in the literature regarding maternal GBS carrier status, but the data remain controversial: the research conducted by Tano et al. demonstrated a noteworthy variation in the prevalence of GBS colonisation between neonates with preterm premature rupture of membranes (PROM) and full-term neonates [21]. Other risk factors reported are obesity, diabetes, advanced maternal age, and black ethnicity [22, 23]. Numerous studies have also demonstrated a higher incidence of GBS colonisation in neonates born to mothers over the age of 30 [24-26]. At the opposite an Italian cohort study shows non-statistically significant excesses noted in pregnant women with term births, those aged over 30 years, Italians, pregnant women residing in rural areas within the province, and multiparous women and GBS colonisation. Additionally, no notable disparities were found between infants born to mothers positive or negative for GBS concerning delivery method, gestational age, or the presence of congenital abnormalities. Similarly, there were no notable increases in stillbirths, Apgar scores below 7 at 5 minutes, or instances of hospitalization at birth [27]. In our study, the distribution of positive cases seems to vary across the diverse categories of pregnant women under examination, suggesting no significant statistical disparities among those with or without a partner, among multiparous women, among Italian citizens, or across various age ranges. However, a notable statistical excess is evident exclusively among mothers with lower levels of education according to data already present in the literature [22]. Indeed, maternal colonisation, depending on geographical area, varies between 10-30% in the US and from 6.5 to 36% in Europe. A systematic review conducted in the years 1996-2006 collected data on 21 studies and 24,093 women from 13 European countries showing prevalence rates of GBS-carrying mothers ranging as follows: Eastern Europe 19.7-29.3%, Western Europe 11-21%, Scandinavia 24.3-36% and Southern Europe 6.5-32%. [28, 29]. The same survey showed that women from European and Latin American countries had colonisation rates of 21% and 22%, respectively, compared with colonisation rates of 29% for African women and 13% for Asian women [29]. Vaginal and rectal swabs in our population of pregnant women were positive for GBS with a prevalence rate of 12.6% according with the national average, which is about 10-20% [30] (Figure 1): in the North Eastern region of Italy it is estimated as 17.9% [31]. In Piemonte, northern Italy, the average prevalence of pregnant women colonised with GBS was 13.8% [20]. In Perugia, the average prevalence was 11.3% in Campobasso 30.2%, in Friuli Venezia Giulia 19.7%, in Palermo 7.98%, in Pistoia 25.5% [19, 32-35]. In Trento the average proportion of positive swab cases during the study period was 21.0% [27] Additionally, in our study, none of our neonates developed EOD or LOD, consistent with the low number of samples of infants born to carrier mothers and the overall incidence of invasive GBS infections. A meta-analysis conducted across 53 countries revealed that approximately 6,199 neonates experienced invasive GBS disease out of 13,300,000 live births, resulting in an overall incidence rate of 0.49 per 1,000 live births, with a range of 0.21 to 2.00 per 1,000 live births [5]. In an Italian cohort study of postnatal evaluation of 783 live births from a GBS positive mother identified 3 cases of early neonatal GBS infection and no cases of late neonatal infection. These three cases were inadequately treated with ampicillin with an observed incidence of neonatal GBS infection over the whole series of live births was 0.71/1,000 in Italians and 1.07/1000 in foreigners [27]. Although numerous evidences have demonstrated that IAP significantly reduces the incidence of EOD [5, 6, 36, 37], it remains to be clarified the correct adherence to the indications for IAP [6]. The research conducted by Piffer et al showed that IAP was performed in 86.8% of births from GBS positive mothers who had an indication. [27]. In Campobasso, Italy, only 50% of women identified as needing IAP received the appropriate treatment [19].
Table 4 - Neonatal outcomes in newborn to GBS positive mothers vs newborn to GBS negative mothers.
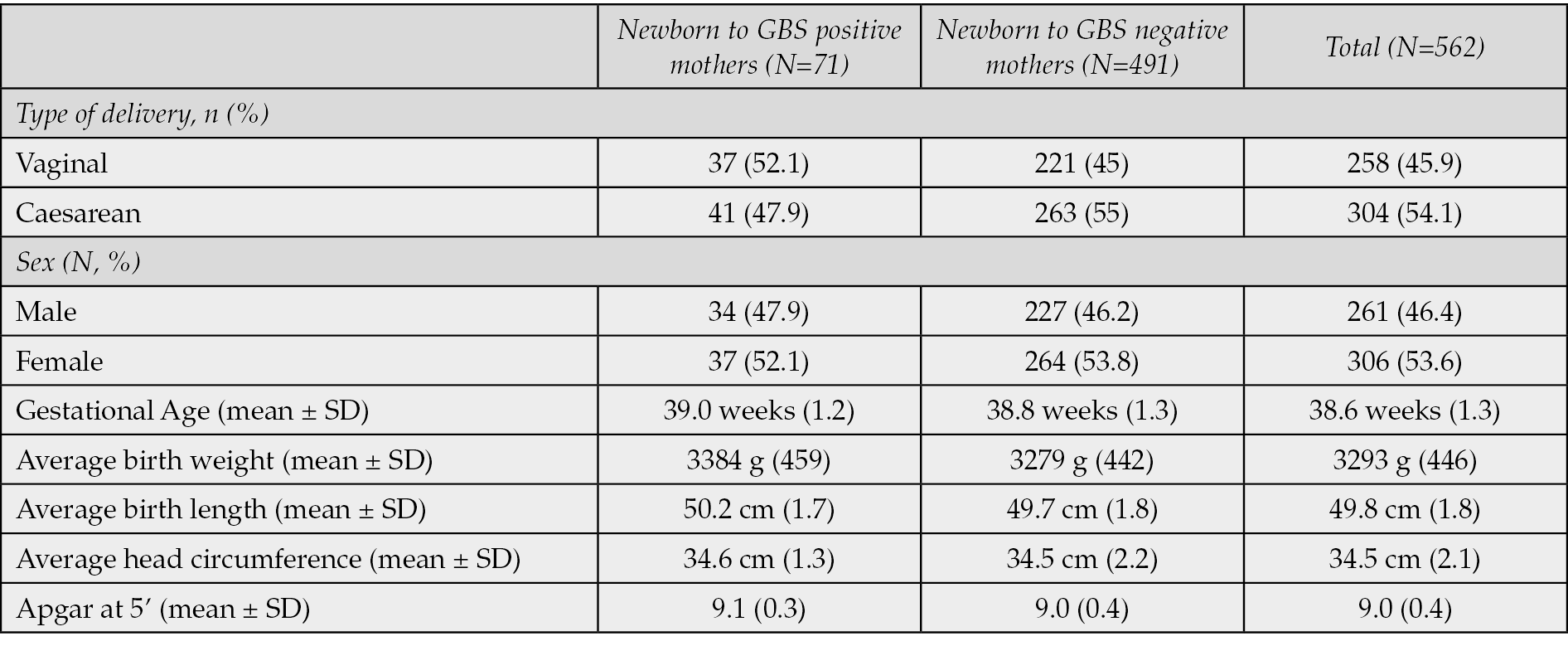
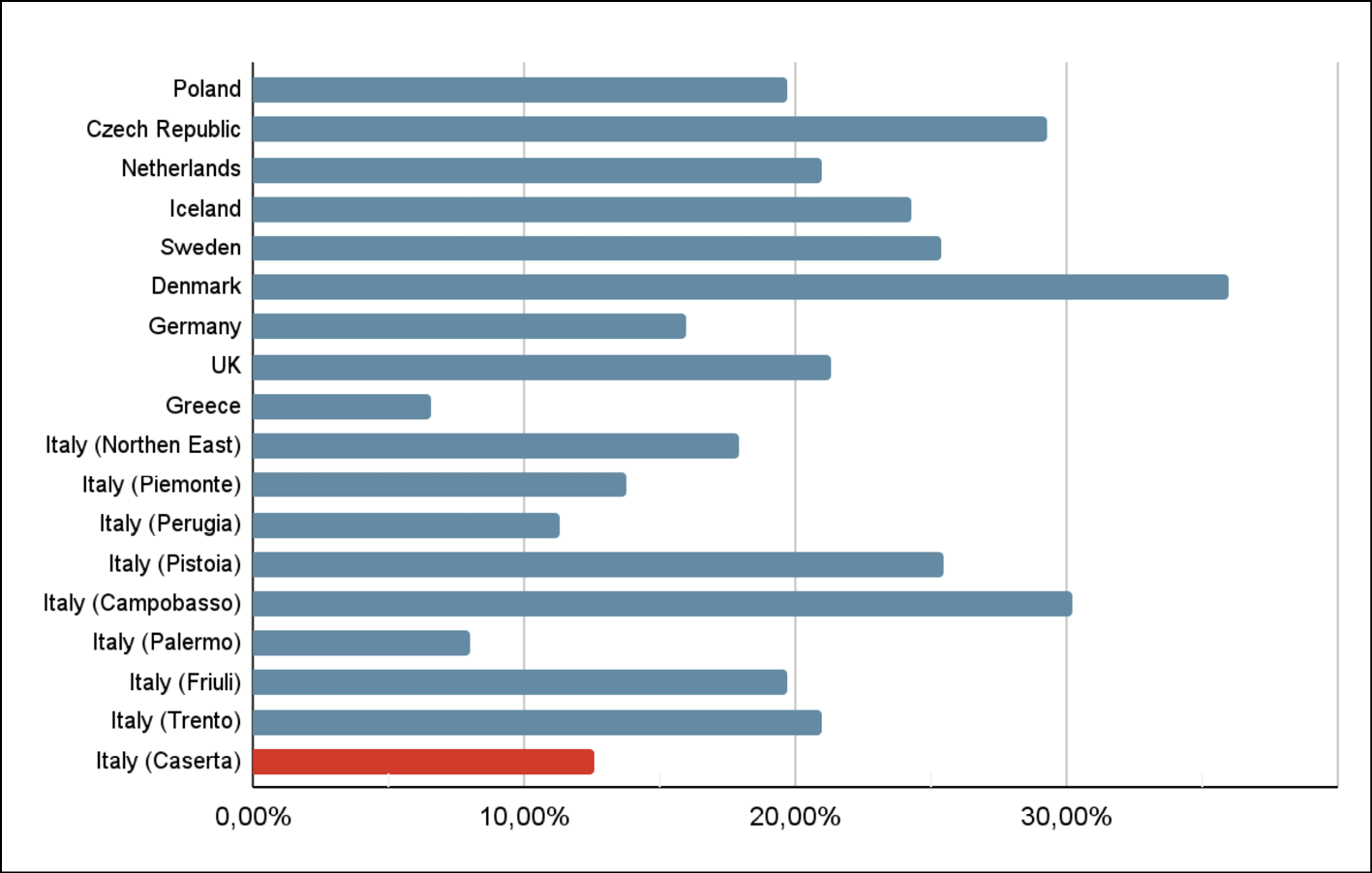
Figure 1 - Maternal GBS colonization rates in various areas, in red our study [19, 20, 27, 29, 32-35].
Meanwhile there is a growing focus on identifying biomarkers capable of detecting early GBS infection for those who are not eligible to VRS screening, such as preterm birth, or IAP, as in precipitous birth. One promising approach involves assessing acute-phase reactant biomarkers in umbilical cord blood at birth, like procalcitonin and activated protein C. This method can improve the identification of early-onset disease in extremely premature infants and is also suitable for monitoring antibiotic therapy. Although these biomarkers exhibit low sensitivity, their efficacy is enhanced when used in combination [38-40]. They also represent the most appropriate biomarkers for monitoring antibiotic therapy [41]. A panel of biomarkers for sepsis would enable early identification, proper management, and enhanced outcomes, potentially proving more effective than a singular indicator. The development of a vaccine against the capsular polysaccharide component of GBS that can be administered during the third trimester of pregnancy is a promising new frontier. An Italian research group demonstrated that optimised virus-like particles, which self-assemble and are conjugated to the capsular polysaccharides of Group B Streptococcus, were able to elicit an adequate immune response in mice after a single administration. Moreover, the protective antibodies generated can cross the placental barrier and provide immunity to the newborn, which provides a foundation for the development of a future human vaccine [42].
CONCLUSIONS
Our findings demonstrate comparable GBS colonisation incidence during pregnancy to that reported in other literature populations. The incidence of GBS-positive women during pregnancy varies worldwide, and our sample from southern Italy indicates a prevalence rate of 12.6%. Because maternal GBS colonisation contributes significantly to neonatal morbidity and mortality, the 2019 guidelines from ACOG and AAP stress the indication for screening pregnant women with VRS during the 36th and 37th weeks of gestation. This screening can identify those who may benefit from intrapartum antibiotic therapy that can curtail early-onset GBS infection. However, despite the successful implementation of GBS screening, there remains a considerable disparity between the ideal and the actual administration of intrapartum antibiotic prophylaxis (IAP). Therefore, the development of vaccination during pregnancy that can provide immunity to the newborn represents a new and promising opportunity. Thus, education of pregnant women regarding the significance of GBS screening and treatment is important, but it is equally and perhaps indispensable that health services put into practice what is recommended by the guidelines, as well as verifying it in practice. It is crucial to carry out fresh investigations on larger and more diverse samples to comprehend the efficacy of invasive GBS infection control and treatment methods, recognize barriers to compliance with culture screening, and investigate the variables that contribute to maternal GBS colonisation variability.
Ethical approval
This article does not contain any studies involving human participants performed by any of the authors. The data used in this study is de-identified from national inpatient data thus informed consent or IRB approval was not needed for this study.
Conflict of interest
All authors declare no conflicts of interest to be disclosed.
Funding
None.
REFERENCES
[1] Schrag SJ, Farley MM, Petit S, et al. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics. 2016; 138(6): e20162013.
[2] Masroori EA, Uraba WB, Al Hashami H. Incidence and outcome of group B streptococcal invasive disease in Omani infants. Int J Pediatr Adolesc Med. 2020; 7(3): 136-139.
[3] Barton LL, Feigin RD, Lins R. Group B beta hemolytic streptococcal meningitis in infants. J Pediatr. 1973; 82(4): 719-723.
[4] Benitz WE, Gould JB, Druzin ML. Antimicrobial prevention of early-onset group B streptococcal sepsis: estimates of risk reduction based on a critical literature review. Pediatrics. 1999; 103(6): e78.
[5] Madrid L, Seale AC, Kohli-Lynch M, et al. Infant GBS Disease Investigator Group. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017; 65 (Suppl. 2): S160-S172.
[6] Creti R, Imperi M, Berardi A, et al. The Italian Network on Neonatal and Infant GBS Infections. Invasive Group B Streptococcal Disease in Neonates and Infants, Italy, Years 2015-2019. Microorganisms. 2021; 9(12): 2579.
[7] Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010; 59(RR-10): 1-36.
[8] Centers for Disease Control and Prevention (CDC). Adoption of perinatal group B streptococcal disease prevention recommendations by prenatal-care providers-Connecticut and Minnesota, 1998. MMWR Morb Mortal Wkly Rep. 2000; 49(11): 2228-2232.
[9] Di Renzo GC, Melin P, Berardi A, et al. Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med. 2015; 28(7): 766-782.
[10] Bianco A, Larosa E, Pileggi C, Pavia M. Collaborative Working Group. Appropriateness of intrapartum antibiotic prophylaxis to prevent neonatal Group B Streptococcus disease. PLoS One. 2016; 11(11): e0166179.
[11] Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obstet Gynecol. 2020 Feb; 135(2): e51-e72. Erratum in: Obstet Gynecol. 2020 Apr; 135(4): 978-979.
[12] Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013; 31 (Suppl. 4): D20-26.
[13] Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019; 134 (1): 1.
[14] Hansen SM, Uldbjerg N, Kilian M, Sørensen UB. Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J Clin Microbiol. 2004; 42(1): 83-89.
[15] Mousavi SM, Hosseini SM, Mashouf RY, Arabestani MR. Identification of Group B Streptococci Using 16S RRNA, Cfb, ScpB, and Atr Genes in Pregnant Women by PCR. Acta Med. Iran. 2016; 54: 765-770.
[16] Carrillo-Ávila JA, Gutiérrez-Fernández J, González-Espín AI, García-Triviño E, Giménez-Lirola LG. Comparison of qPCR and culture methods for group B Streptococcus colonization detection in pregnant women: evaluation of a new qPCR assay. BMC Infect Dis. 2018; 18(1): 305.
[17] Hayes K, O’Halloran F, Cotter L. A review of antibiotic resistance in Group B Streptococcus: the story so far. Crit Rev Microbiol. 2020; 46(3): 253-269.
[18] El Beitune P, Duarte G, Maffei C. Colonization by Streptococcus agalactiae during pregnancy: maternal and perinatal prognosis. Braz. J. Inf. Dis. 2005; 9: 276-282.
[19] De Luca C, Buono N, Santillo V, et al. Screening and management of maternal colonization with Streptococcus agalactiae: an Italian cohort study. J Matern Fetal Neonatal Med. 2016; 29(6): 911-915.
[20] Tibaldi C, Masuelli G, Mischinelli M, Sola I, Todros T, Prevenzione dell’infezione neonatale da streptococco β emolitico di gruppo B (GBS). Risultati del Progetto Regionale di Ricerca finalizzata sui protocolli in uso presso i punti nascita e proposta di un protocollo regionale condiviso, in “Tendenze nuove, Materiali di lavoro su sanità e salute della Fondazione Smith Kline” 2/2010, pp. 141-148, Available at: https://www.rivisteweb.it/doi/10.1450/31940 [accessed 12, October, 2023].
[21] Tano S, Ueno T, Mayama M, et al. Relationship between vaginal group B streptococcus colonization in the early stage of pregnancy and preterm birth: a retrospective cohort study. BMC Pregnancy Childbirth. 2021; 21(1): 141.
[22] Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal Infections and Prematurity Study Group. Obstet Gynecol. 1991; 77(4): 604-610.
[23] Venkatesh KK, Vladutiu CJ, Strauss RA, et al. Association Between Maternal Obesity and Group B Streptococcus Colonization in a National U.S. Cohort. J Womens Health (Larchmt). 2020; 29(12): 1507-1512.
[24] Schuchat A, Deaver-Robinson K, Plikaytis B, Zangwill K, MohleBoetani J, Wenger JD. Multistate case-control study of maternal risk factors for neonatal group B Streptococcal disease. The active surveillance study group. Pediatr. Infect. Dis. 1994; 13: 623-629.
[25] Al-Kadri Hanan M, Bamuhair Samira S, Al Johani Sameera M, Al-Buriki Namsha A, Tamim Hani M. Maternal and neonatal risk factors for early-onset group B streptococcal disease: a case control study. Int. J. Womens Health. 2013; 2013(5): 729-735.
[26] Al Hazzani AA, Bawazeer RAB, Shehata AI. Epidemiological characterization of serotype group B Streptococci neonatal infections associated with interleukin-6 level as a sensitive parameter for the early diagnosis. Saudi J Biol Sci. 2018; 25(7): 1356-1364.
[27] Piffer S, Rizzello R, Pedron M, Dellanna L, Lauriola AL. Screening of group B Streptococcus infection in pregnancy and neonatal outcomes in the province of Trento, Italy. Infez Med. 2022; 30(2): 254-262.
[28] Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002; 51(RR-11): 1-22.
[29] Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R. Prevalence of maternal group B streptococcal colonisation in European countries. Acta Obstet Gynecol Scand. 2008; 87(3): 260-271.
[30] Infezioni neonatali precoci e tardive da streptococco di gruppo B in Italia. Edited by Creti R. Roma: Istituto Superiore di Sanità; 2011. Rapporti ISTISAN 11/7. Available at: https://www.iss.it/documents/20126/45616/undici7web.pdf/f03bbefd-897e-1d45-ba73-ed675b2d3789?t=1581099726222 [accessed 18, October, 2023].
[31] Busetti M, D’Agaro P, Campello C. Group B streptococcus prevalence in pregnant women from North-Eastern Italy: advantages of a screening strategy based on direct plating plus broth enrichment. J Clin Pathol. 2007; 60(10): 1140-1143.
[32] Sensini A, Tissi L, Verducci N, et al. Carriage of group B Streptococcus in pregnant women and newborns: a 2-year study at Perugia General Hospital. Clin Microbiol Infect. 1997; 3(3)3] Cantoni L, Ronfani L, Da Riol R, Demarini S: Physical examination instead of laboratory tests for most infants born to mothers colonized with Group B Streptococcus: Support for the Centers for Disease Control and Prevention’s 2010 Recommendations. J Pediatr. 2013, 163: 568-573.
[33] Puccio G, Cajozzo C, Canduscio LA, et al. Epidemiology of Toxoplasma and CMV serology and of GBS colonization in pregnancy and neonatal outcome in a Sicilian population. Ital J Pediatr. 2014; 40, 23.
[34] Matani C, Trezzi M, Matteini A, Catalani C, Messeri D, Catalani C. Streptococcus agalactiae: prevalence of antimicrobial resistance in vaginal and rectal swabs in Italian pregnant women. Infez Med. 2016; 24(3): 217-221.
[35] Centers for Disease Control and Prevention (CDC). Perinatal group B streptococcal disease after universal screening recommendations - United States, 2003-2005. MMWR Morb Mortal Wkly Rep. 2007; 56(28): 701-705.
[36] Centers for Disease Control and Prevention (CDC). Trends in perinatal group B streptococcal disease - United States, 2000-2006. MMWR Morb Mortal Wkly Rep. 2009; 58(5): 109-112.
[37] Frerot A, Baud O, Colella M, et al. Cord blood procalcitonin level and early-onset sepsis in extremely preterm infants. Eur J Clin Microbiol Infect Dis. 2019; 38(9): 1651-1657.
[38] Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit Care. 2018; 22(1): 316.
[39] Eschborn S, Weitkamp JH. Procalcitonin versus C-reactive protein: review of kinetics and performance for diagnosis of neonatal sepsis. J Perinatol. 2019; 39(7): 893-903.
[40] Memar MY, Alizadeh N, Varshochi M, Kafil HS. Immunologic biomarkers for diagnostic of early-onset neonatal sepsis. J Matern Fetal Neonatal Med. 2019; 32(1): 143-153.
[41] Carboni F, Cozzi R, Romagnoli G, et al. Proof of concept for a single-dose Group B Streptococcus vaccine based on capsular polysaccharide conjugated to Qβ virus-like particles. NPJ Vaccines. 2023; 8(1): 152.