Le Infezioni in Medicina, n. 2, 202-212, 2024
doi: 10.53854/liim-3202-8
ORIGINAL ARTICLES
The prevalence of long COVID-19 syndrome in hospitalized patients with COVID-19 pneumonia
Vasileios Petrakis1, Petros Rafailidis1, Irene Terzi1, Ioulia Dragoumani1, Filothei Markatou1, Nikolaos Papanas1, Stergios Vradelis1, Evanthia Gouveri1, Maria Panopoulou2, Dimitrios Papazoglou1, Panagopoulos Periklis1
1Department of Infectious Diseases, 2nd University Department of Internal Medicine, University General Hospital Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece;
2University Laboratory Department, University General Hospital Alexandroupolis, Democritus University of Thrace, Alexandroupolis, Greece
Article received 18 February 2024, accepted 14 April 2024
Corresponding author
Vasileios Petrakis
E-mail: vasilispetrakis1994@gmail.com
SummaRY
Introduction: Long COVID affects millions of individuals worldwide with a wide range of persistent symptoms. Pathogenesis, prevalence and clinical approach of this syndrome remain not well characterized.
The aim of the study is the estimation of prevalence of long-COVID and identification of possible risk factors.
Patients and Methods: This is an observational prospective study including COVID-19 patients hospitalized at the Department of Infectious Diseases of the University General Hospital of Alexandroupolis (Greece). Eligible COVID-19 patients were interviewed and examined 6, 12 and 18 months after COVID-19 symptoms onset and hospital discharge in order to evaluate the prevalence and consequences of long-COVID symptoms.
Results: A total number of 995 patients were included. The median age at discharge was 55 years and 53% of patients were retired. The majority was males (57%). Vaccination against SARS-CoV-2 was completed in 52% (n=517) COVID-19 patients. More than 40% of COVID-19 patients had at least one symptom at 18 months after hospitalization. Intravenous antiviral treatment with remdesivir and complete vaccination status were found to lead to lower rates of Long-COVID.
Conclusions: More studies in larger patient cohorts are needed in order to identify the underlying biological mechanisms of long-COVID and create effective interventions for prevention and treatment.
Keywords: SARS-CoV-2, COVID-19, Long-COVID.
INTRODUCTION
As of December, 2023, more than 770 million confirmed cases of the coronavirus disease 2019 (COVID-19) have been reported, caused by the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) [1]. Although the SARS-CoV-2-infection causes mild symptoms to the majority of infected patients, a small portion can progress to severe COVID-19 or acute respiratory distress syndrome, especially in patients with comorbidities such as cardiovascular disease, obesity, diabetes mellitus, malignancies, immunosuppression and chronic renal disease [2]. The vaccination and efficient orally or intravenously administered antiviral agents were managed to limit the severity of acute illness leading to lower probability of intubation, prolonged hospitalization and severe clinical outcome [3, 4]. However, a considerable number of patients may experience persistent symptoms over weeks, months and even years, including general tiredness, muscle pain, difficulties of breathing, tingling extremities or chest pain after the acute phase of COVID-19 disease, a condition characterized as long COVID-19 syndrome [5]. In 2021, a WHO working group put in place a Delphi consensus definition of long COVID as a condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis [6]. UK National Institute for Health and Care Excellence (NICE) defined Long COVID as a multisystem condition with a range of debilitating symptoms continuing or developing after acute COVID-19 for more than 4 weeks without any alternative diagnosis [7]. This definition includes both ongoing symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more) [7].
The estimation of the incidence rate of long COVID-19 syndrome is difficult due to the use of different methodologies and definitions [6]. In published studies, the frequency of long COVID-19 varies from below 10% to around 60% [8-11]. A Scottish population cohort of 33,281 laboratory-confirmed SARS-CoV-2 infections was followed up via 6, 12 and 18-month questionnaires and linkage to hospitalization and death records [10]. Of the 31,486 symptomatic infections, 1,856 (6%) had not recovered and 13,350 (42%) only partially [10]. Previous symptomatic infection was associated with poorer quality of life, impairment across all daily activities and several persistent symptoms including breathlessness (OR 3.43, 95% CI 3.29-3.58), palpitations (OR 2.51, OR 2.36-2.66), chest pain (OR 2.09, 95% CI 1.96-2.23), and confusion (OR 2.92, 95% CI 2.78-3.07) [10]. A systematic literature search including 194 studies and 735,006 participants has documented that 45% of COVID-19 survivors, regardless of hospitalisation status, were experiencing a range of unresolved symptoms at ∼ 4 months [11]. Although the rates of complete vaccination status are high and the presentations of acute infection are milder during recent variant waves, the cumulative burden of long COVID remains significant and increasing [6].
The primary objectives of the present study are to evaluate the quality of health, the functional status and the prevalence of Long COVID symptoms in COVID-19 survivors hospitalized in the Department of Infectious Diseases of University General Hospital Alexandroupolis (Greece) up to almost 2 years after acute infection. The exact number of people living with Long COVID worldwide remains unknown and thus, reports of real-world data are significant to underline the impact of the syndrome on the quality of health and life. Long COVID-19 syndrome has been characterized as a fourth phase of SARS-CoV-2 infection and understanding the prevalence and clinical manifestations of this new medical condition is vital for a holistic, multidisciplinary and patient centered approach [12].
PATIENTS AND METHODS
This is an observational cohort study conducted in the Department of Infectious Diseases of the University General Hospital of Alexandroupolis (Greece). COVID-19 patients hospitalized with COVID-19 pneumonia from 1st January 2021 to 31st May 2022 were included in the study after written informed consent. The study was carried out in accordance with the Helsinki Declaration of Human Rights. COVID-19 patients who died before the follow-up visits, living in a nursing or welfare home and had difficulty completing the visits due to mental disorders or dementia were excluded.
Eligible COVID-19 patients were interviewed and examined 6, 12 and 18 months after COVID-19 symptoms onset and hospital discharge at the outpatient clinic of our department. Demographic characteristics, comorbidities, data of hospitalization (oxygen therapy, intubation, duration of hospitalization, antiviral treatment) during acute illness and long-term health consequences were retrieved from electronic medical records. As acute phase was defined the period between symptoms onset and hospital discharge. The vaccination status during acute phase was reported and assessed according to National Guidelines of National Public Health Organisation during the period 2021-2022 (complete vaccination scheme and one booster dose) [13]. They were categorised into three groups based on severity of symptoms during the actute infection (scale 3: not requiring supplemental oxygen; scale 4: requiring supplemental oxygen; scale 5-6: requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation) [14]. Blood samples were collected at each visit to evaluate the white blood cell count, platelets count, haemoglobin and haematocrit values, liver function, blood glucose levels, renal function, lipid profile and cytokines levels. Chest computed tomography was conducted at each visit in order to assess the progress of lesions due to COVID-19 pneumonia. The exercise capacity was evaluated by the 6-minute walking test (6MWD). Lung function parameters including forced expiratory volume in one 1 second (FEV1), forced vital capacity (FVC), total lung capacity (TLC), functional residual capacity (FRC), residual volume (RV), and diffusion capacity for carbon monoxide (DLCO) were measured at first and last visit in authorised Pulmonology outpatient clinics. The quality of sleep was evaluated with the questionnaires Fatigue Severity Scale (FSS), Sleep Quality Scale (MOS) and Epworth Sleepiness scale (ESS), while the prevalence of psychological symptoms with the Hospital Anxiety and Depression Scale (HADS). These questionnaires are validated to the Greek population [15-18]. All patients were questioned at all visits about symptoms such as fatigue, muscle weakness, sleep difficulties, hair loss, smell disorder, palpitations, joint pain, taste disorder, dizziness, nausea, vomiting, chest pain, sore throat, swallow difficulties, skin rash, myalgia, headache and persistent cough. These symptoms were attributed to long-COVID if there was no alternative diagnosis during the diagnostic process and medical assessment. Cardiovascular events and possible hospitalizations due to possible long-COVID symptoms during the study period were recorded. Work status was also documented. Symptoms newly occurring and persistent after acute infection, which cannot be explained by an alternative disease, were characterized as sequelae symptoms. COVID-19 participants with at least one sequelae symptom at follow-up visits were defined as patients with long-COVID symptoms.
The primary outcomes were the sequelae symptoms existing at follow-up visits without any alternative diagnosis, the exercise capacity assessed by 6MWD, and return to work. The secondary outcomes were lung function, imaging (Chest CT), cardiovascular events and hospitalizations after discharge. Statistical analysis of the data was performed using the IBM Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM Corp., Armonk, NY, USA). The normality of quantitative variables was tested using the Kolmogorov–Smirnov test. Normally, distributed quantitative variables were expressed as the mean ± standard deviation (SD), whereas non-normally distributed quantitative variables are expressed as the median value and range. Qualitative variables were expressed as absolute and relative (%) frequencies. Student’s t-test, the Mann–Whitney U test and the chi-square test were used to determine differences in demographic and clinical characteristics. All tests were two-tailed and p values <0.05 were considered statistically significant.
RESULTS
A total number of 995 patients hospitalized with confirmed COVID-19 disease during the period 1st January 2021 to 31st May 2022 at the Department of Infectious Diseases of the University General Hospital of Alexandroupolis (Greece) were eligible and included in the study (Figure 1). The median age at discharge was 55 years and 53% of patients were retired. The majority was males (57%). Vaccination against SARS-CoV-2 was completed based on National Guidelines in 52% (n=517) of COVID-19 patients when infected. The most frequent comorbidities were hypertension (35%), diabetes mellitus (16%) and coronary heart disease (11%). Three groups were created based on the severity of COVID-19 symptoms during acute phase of infection [14]. No supplemental oxygen was required for 206 patients (scale 3), while oxygen therapy was needed for 611 patients (scale 4). Severe clinical progress requiring high-flow nasal cannula, non-invasive mechanical ventilation, or invasive mechanical ventilation was reported in 178 patients (scale 5-6). Demographic and clinical characteristics of COVID-19 patients are shown in Table 1. The major treatment received during hospitalization was antimicrobial and antiviral agents. Among patients with severe clinical progress (scale 5-6) the administration of intravenous corticosteroids and immunomodulators was high, 76% and 94% respectively (Table 2).
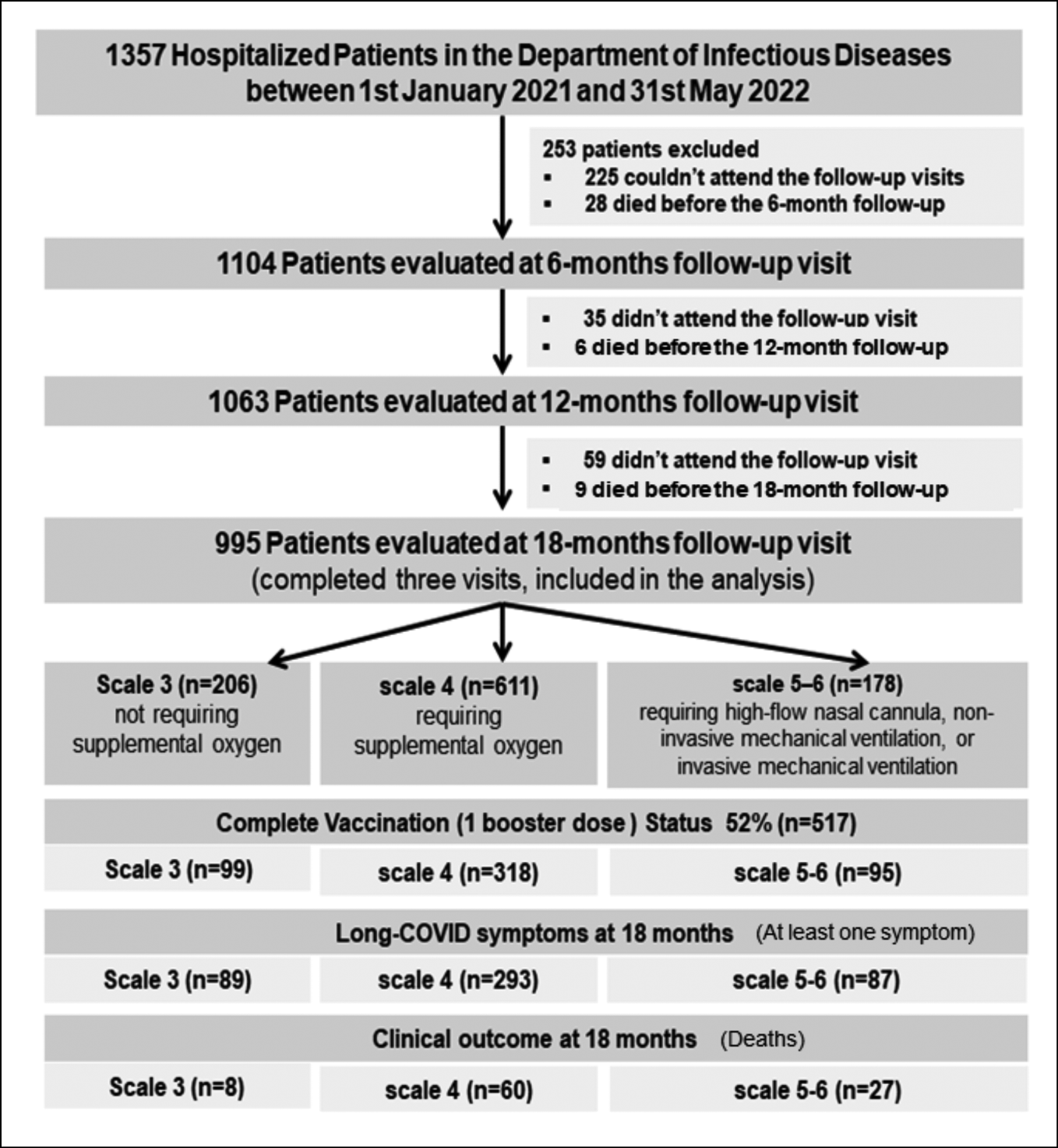
Figure 1 - Flowchart of the study.
Table 1 - Demographic and clinical characteristics of COVID-19 patients.

Table 2 - Hospitalization data during acute phase of COVID-19 disease.
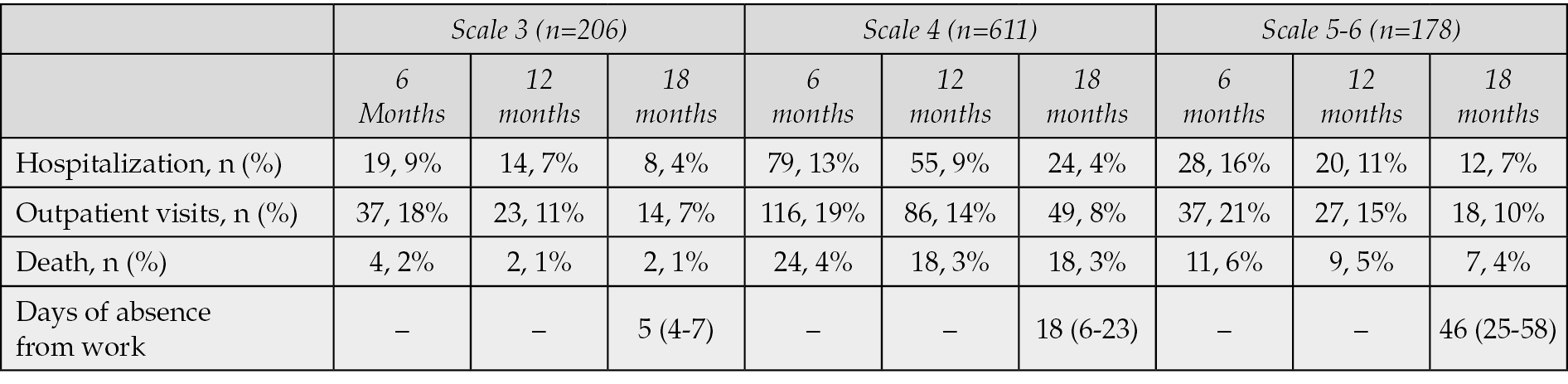
More than 40% of COVID-19 patients had at least one symptom at 18 months after hospitalization (scale 3: 43%, scale 4: 48%, scale 5-6: 49%) (Table 3, Figure 2). The most frequent reported symptoms were fatigue, muscle or joints pains, symptoms of depression and sleep disorders such as insomnia or restless leg syndrome (Figure 2). Other common symptoms without associated alternative diagnosis were palpitations, brain fog, hair loss, skin rashes and chest pain (Figure 2). Chest computed tomography abnormalities remained in approximately 60% of COVID-19 patients on scale 5-6 at 18 months. The most frequent findings were the ground glass opacity, the reticular pattern, the consolidations and the interlobular septal thickenings. Although the median 6MWD was lower for patients with more severe acute illness, the values were significantly improved at 18 months for all the subgroups. More than 50% of patients with severe COVID-19 had abnormal pulmonary function tests at 18 months with reduced total lung capacity. The results of the evaluation of pulmonary function are presented in Table 4. The number of days of absence from work was significantly higher for patients on scale 5 or 6 (Table 5). Hospitalization due to complications related to long COVID-19 symptoms was documented in about 10% of patients of all subgroups (Table 5). The highest mortality rate was observed in patients who needed high-flow nasal cannula or mechanical ventilation during acute illness (4% at 18 months, scale 5-6, Table 5).
Table 3 - Sequelae symptoms associated with Long-COVID syndrome.
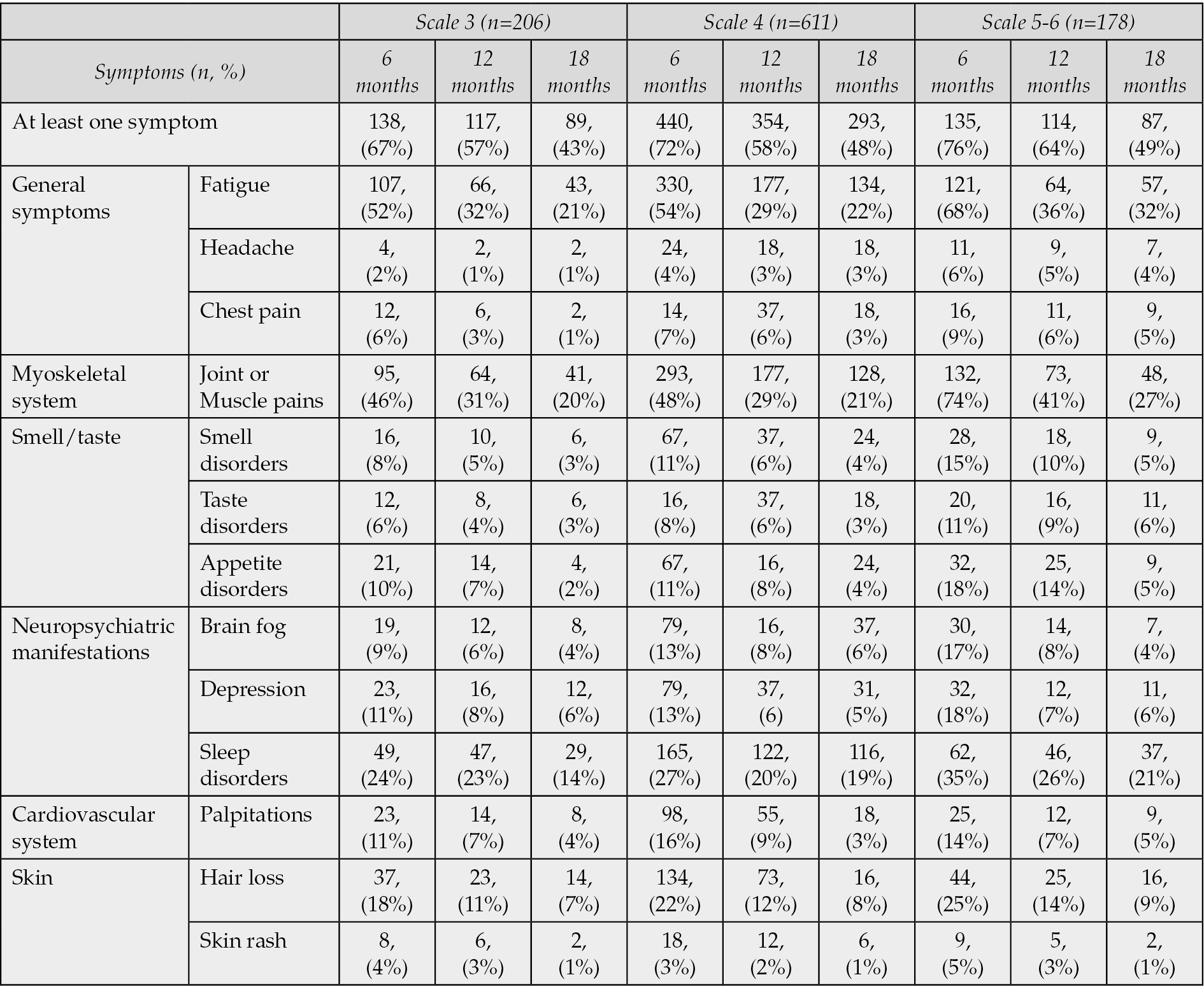
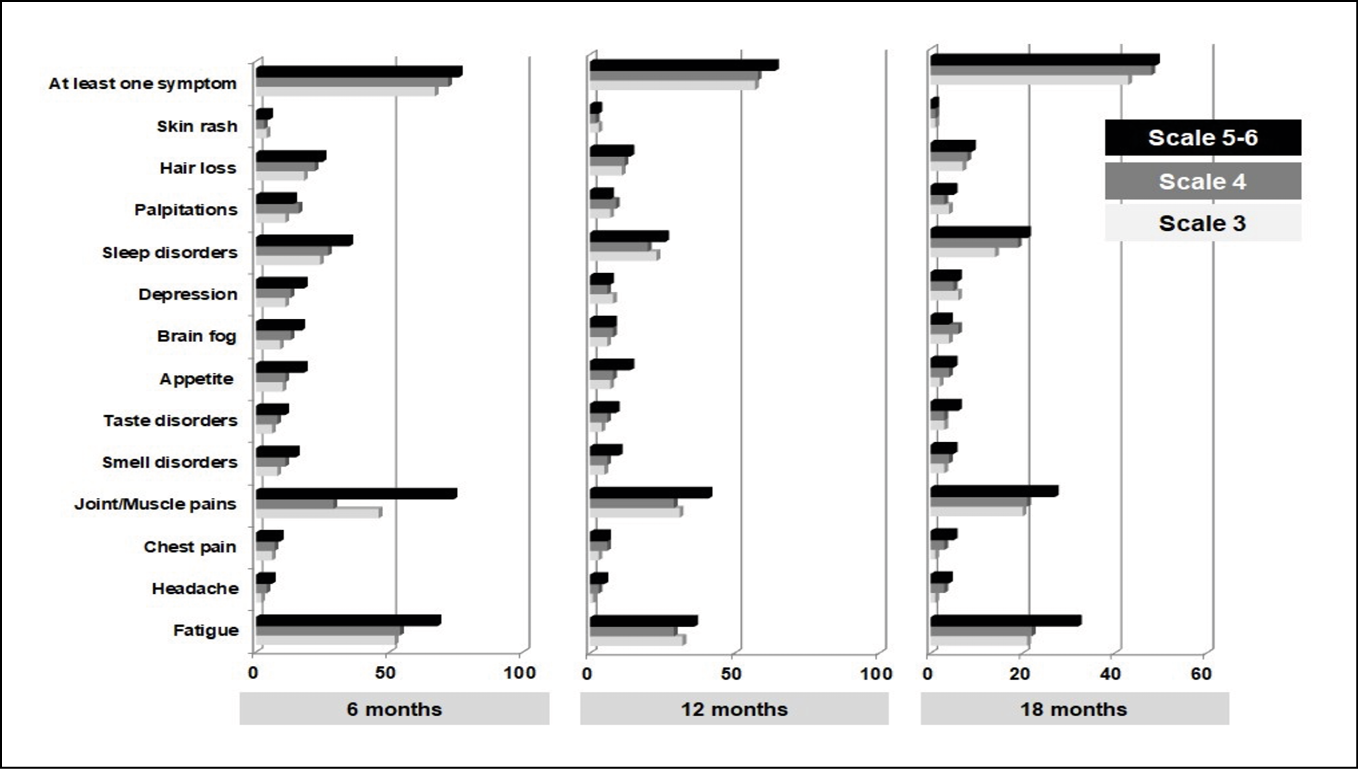
Figure 2 - Persistent long-COVID symptoms in COVID-19 patients.
Table 4 - Evaluation of pulmonary function of COVID-19 patients.
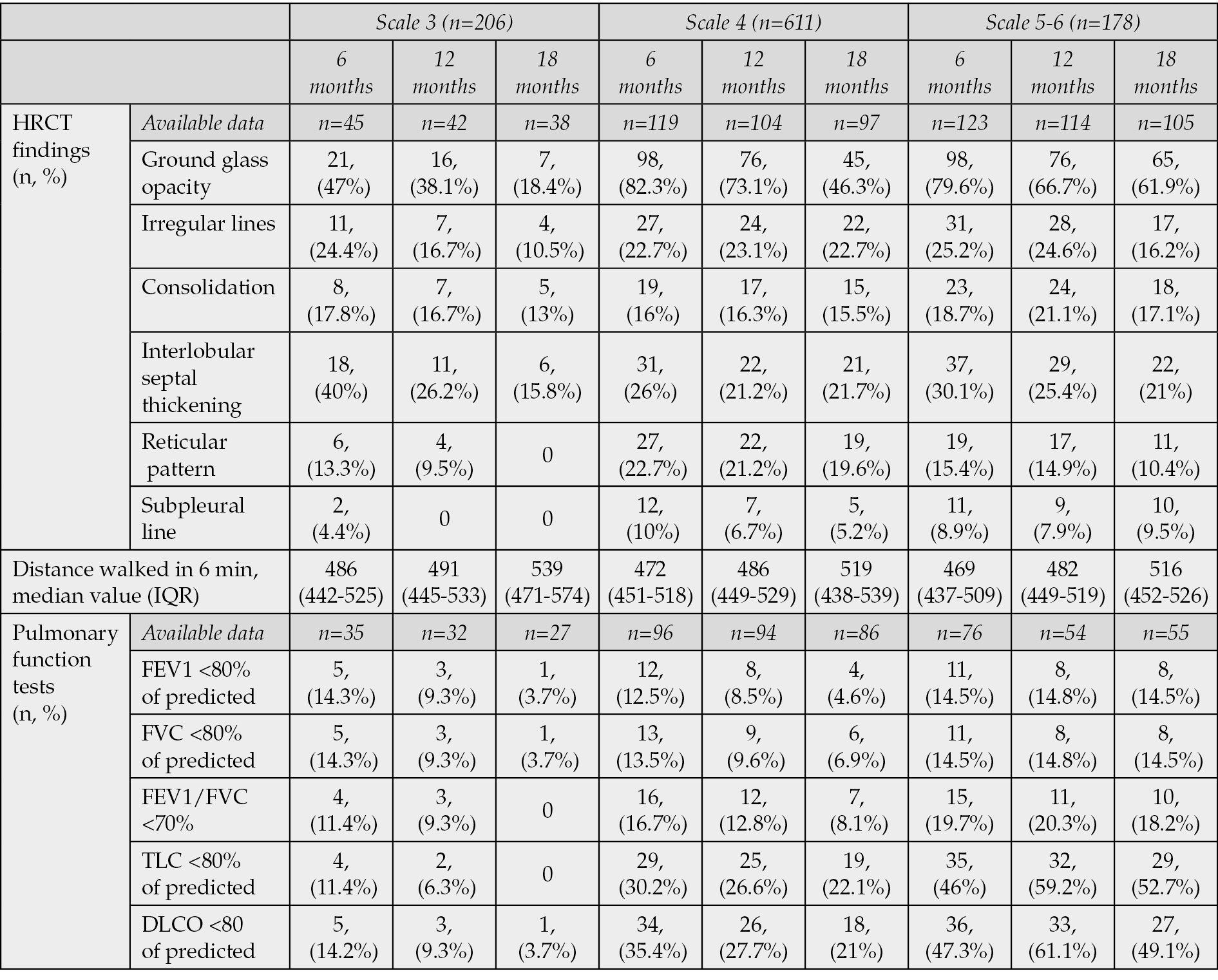
Table 5 - Clinical outcome of patients with Long-COVID symptoms.
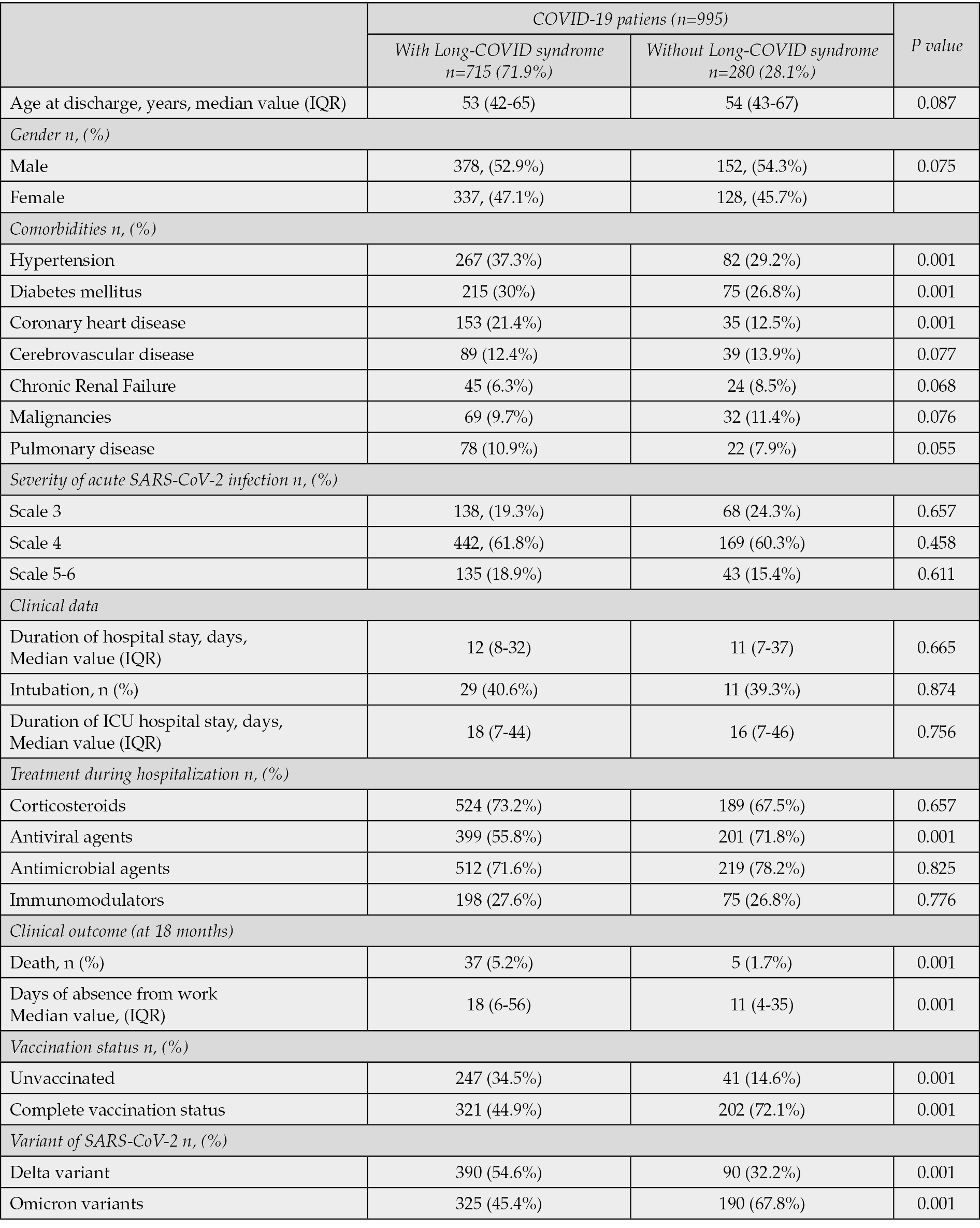
The results of multivariable logistic regression analysis indicated that increased age (>60 years old), male gender, unvaccinated, presence of comorbidities, eventually diabetes mellitus and infection with Delta variant are associated with a higher risk of persistent long-COVID symptoms (Table 6). Intravenous antiviral treatment with remdesivir and complete vaccination status were found to lead to lower rates of Long-COVID (Table 6). Among 995 COVID-19 patients, 280 (28.1%) individuals had no long-COVID symptoms at 6-, 12- and 18-months follow-up vistis (Table 7). Demographic and clinical data were similar with patients with long-COVID syndrome. However, treatment with antiviral agents, comorbidities such as diabetes mellitus, complete vaccination status and infection with Omicron variants were found in higher rates in patients without long-COVID syndrome supporting the findings of multivariable analysis (Table 7).
Table 6 - Mulivariable logistic regression analysis. Risk factors of long-COVID symptoms.
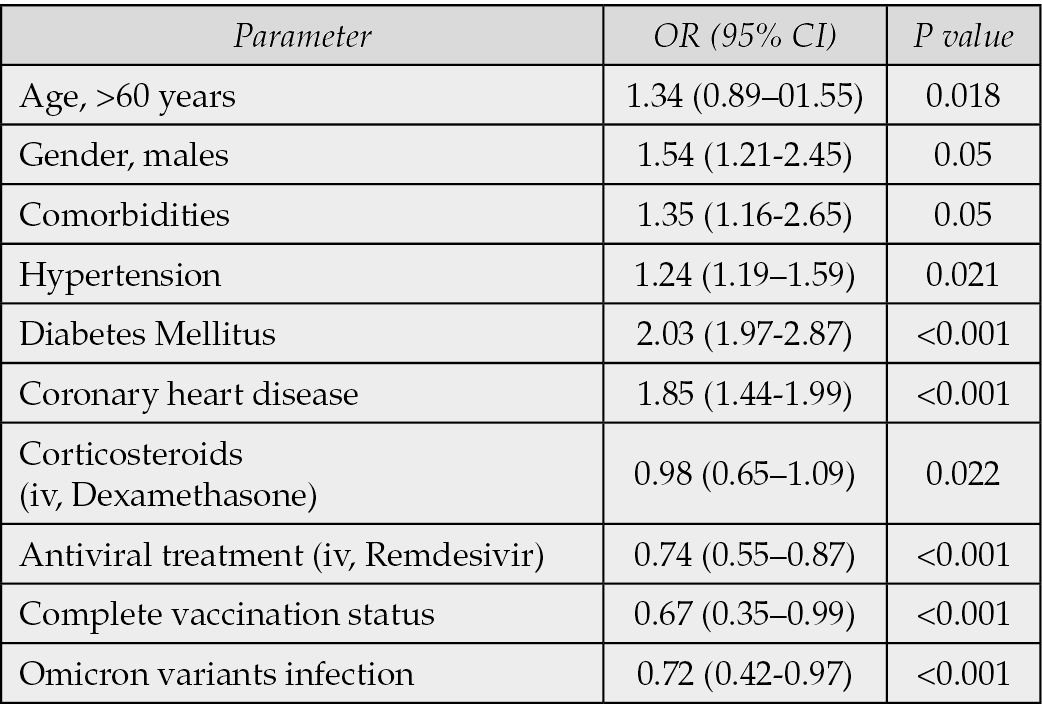
Table 7 - Demographic and clinical data, vaccination status and variants of SARS-CoV-2 among COVID-19 patients with or without long-COVID syndrome.
DISCUSSION
The results of the present study indicate that although longitudinal improvement was observed in clinical status, radiographic findings and pulmonary function tests within 18 months after symptoms onset, the percentage of COVID-19 survivors with at least one persistent symptom remains high at almost two years after acute illness. Early administration of antiviral agents and complete vaccination status were associated with lower risk of long-COVID symptoms. Male gender and comorbidities such as diabetes mellitus were found to increase significantly the risk of long-COVID. The study findings underline the need to analyze and understand the pathogenesis of long COVID in order to develop management strategies for prevention and treatment.
Several studies have been conducted in order to analyze the prevalence and nature of long-COVID-19 [19-27]. A systematic review examining the frequency and variety of persistent symptoms after COVID-19 found that 72,5% of patients had at least one persistent symptom without alternative diagnosis [19]. A multidisciplinary, prospective, population-based, observational cohort study examining the health and health-related behaviours of people living in the north of the Netherlands, assessed the longitudinal dynamics of 23 somatic symptoms surrounding COVID-19 diagnoses among 4231 participants with known SARS-CoV-2 infection [20]. In 12,7% of patients, symptoms such as chest pain, difficulties with breathing, pain when breathing, painful muscles, ageusia or anosmia, tingling extremities and general tiredness, were attributed to COVID-19, as 381 (21,4%) of 1782 COVID-19-positive participants versus 361 (8,7%) of 4130 COVID-19-negative controls had at least one of these symptoms increased to at least moderate severity at 90-150 days after COVID-19 diagnosis [20].
A systematic review and meta-analysis including 81 studies found that the proportion of individuals experiencing fatigue 12 or more weeks following COVID-19 diagnosis was 0.32 (95% CI, 0.27, 0.37; p<0.001; n=25,268; I2=99.1%) [21]. The proportion of individuals with cognitive impairment was 0.22 (95% CI, 0.17, 0.28; p<0.001; n=13,232; I2=98.0) [21]. An online survey of people with suspected (diagnostic/antibody negative or untested; n=2742) and confirmed (diagnostic/antibody positive; n=1020) COVID-19, distributed via COVID-19 support and social media was conducted from September 6, 2020 to November 25, 2020 and analyzed responses from 3762 participants [22]. For the majority of respondents (>91%), the time to recovery exceeded 35 weeks [22].
Studies have shown that the vast majority of patients returned to work after COVID-19 infection, with patients experiencing severe COVID-19 having prolonged time to return to work [23, 24]. COVID-19 patients may need more time for rehabilitation and return to work compared with influenza patients [25]. A retrospective Nationwide Danish registry-based cohort study showed that among 7466 patients, 81.9% (6119/7466) and 98.4% (7344/7466) returned to work within 4 weeks and 6 months, respectively, with 1.5% (109/7466) not returning [25]. More delayed return to work was documented among patients admitted to the ICU while 36% (9/25) did not return within 6 months [25].
A systematic review and meta-analysis of six observational studies involving 536,291 unvaccinated and 84,603 vaccinated (before SARS-CoV-2 infection) patients (mean age, 41.2-66.6; female, 9.0-67.3%) and six observational studies involving 8,199 long COVID patients (mean age, 40.0 to 53.5; female, 22.2-85.9%) who received vaccination after SARS-CoV-2 infection revealed that COVID-19 vaccination before SARS-CoV-2 infection was associated with a lower risk of long COVID [26]. Multicenter observational studies comparing vaccinated COVID-19 patients with unvaccinated, a lower incidence of long COVID was reported among vaccinated patients through 28- to 180-day follow-up [27, 28]. The findings of our study support the positive effect of vaccination on reducing the prevalence of long-COVID symptoms.
A retrospective, propensity-score-matched case–control study demonstrated that early therapy with monoclonal antibodies or antivirals against acute SARS-CoV-2 infection reduces the risk of occurrence of long COVID (11% vs 34% in untreated patients; p=0.001) [30]. In our study, similar findings were reported, as antiviral treatment was significantly associated with lower risk of experiencing long-COVID.
The strengths of our study are the adequate sample size and the longitudinal design with a long follow-up. One limitation of the study is the absence of control group for comparison of our findings with the general population, non-hospitalized COVID-19 patients and/or patient who never reported COVID-19 disease. Additionally, due to the study period, there are patients infected with different variants of SARS-CoV-2 (Delta or Omicron) and the effect of complete vaccination scheme with booster doses, treatment with the oral antiviral agent nirmatrelvir/ritonavir and breakthrough infections or re-infections was not studied.
CONCLUSIONS
Long COVID is a multisystemic illness with persistent symptoms from all organ systems affecting the quality of life of millions of individuals worldwide. More than 50% of COVID-19 survivors experience at least one symptom 2 years after acute illness. Data about the prevalence, pathogenesis, diagnostic approach and clinical management are insufficient, and more studies in larger patient series are vital in order to identify the underlying biological mechanisms and create effective interventions for prevention and treatment.
Author contributions
Conceptualization, VP and PP; methodology, PP; software, VP; formal analysis, VP; investigation, VP, FM, ID, IT, MP, EG, SV; resources, VP; data curation, XX; writing - original draft preparation, VP; writing - review and editing, VP, PP, DP; visualization, VP; supervision, PP; project administration, PP, DP All authors have read and agreed to the published version of the manuscript.
Funding
None.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of University General Hospital of Alexandroupolis and Democritus University of Thrace (protocol code 3978-785/10-12-2021).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
Our research data are available after a request to the corresponding author.
Conflicts of interest
The authors declare no conflict of interest.
REFERENCE
[1] World Health Organization, https://covid19.who.int/ (Access: 9th December 2023).
[2] Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020; 53: 404-412.
[3] Lin KY, Wu PY, Liu WD, et al. Effectiveness of COVID-19 vaccination among people living with HIV during a COVID-19 outbreak. J Microbiol Immunol Infect. 2022; 55: 535-539.
[4] Lai CC, Chao CM, Hsueh PR. Clinical efficacy of antiviral agents against coronavirus disease 2019: a systematic review of randomized controlled trials. J Microbiol Immunol Infect. 2021; 54: 767-775.
[5] Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (Access: 9th December 2023)
[6] Altmann DM, Whettlock EM, Liu S, et al. The immunology of long COVID. Nat Rev Immunol. 2023; l 23: 618-634.
[7] Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021; 9(2): 129.
[8] Lund LC, Hallas J, Nielsen H, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021; 21(10): 1373-1382.
[9] Mizrahi B, Sudry T, Flaks-Manov N, et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ. 2023; 380: e072529.
[10] Hastie CE, Lowe DJ, McAuley A, et al. Outcomes among confirmed cases and a matched comparison group in the Long-COVID in Scotland study. Nat Commun. 2022; 13(1): 5663.
[11] O’Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine. 2022; 55: 101762.
[12] Staffolani S, Iencinella V, Cimatti M, et al. Long COVID-19 syndrome as a fourth phase of SARS-CoV-2 infection. Infez Med. 2022; 30(1): 22-29.
[13] National Guidelines for Vaccination against SARS-CoV-2, National Public Health Organisation (2021, 2022). https://eody.gov.gr/en/ (Access: 23rd March 2024).
[14] Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021; 4 (5): e2111417.
[15] Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000; 48(6): 555-560.
[16] Bakalidou D, Skordilis EK, Giannopoulos S, et al. Validity and reliability of the FSS in Greek MS patients. Springerplus. 2013; 2(1): 304.
[17] Bougia MK, Oikonomou NT, Ntafouli M, et al. AB023. Investigation of the reliability of the Medical Outcomes Study (MOS) Sleep Scale in Greek patients with sleep-related breathing disorders. Ann Transl Med. 2016; 4(22): AB023.
[18] Michopoulos I, Douzenis A, Kalkavoura C, et al. Hospital Anxiety and Depression Scale (HADS): validation in a Greek general hospital sample. Ann Gen Psychiatry. 2007; 7: 4.
[19] Plaçais L, Richier Q, Noël N, et al. Immune interventions in COVID-19: a matter of time? Mucosal Immunol. 2022; 15(2): 198-210.
[20] Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022; 400(10350): 452-461.
[21] Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021; 18.
[22] Ceban F, Ling S, Lui LMW, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun. 2022; 101: 93-135.
[23] Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021; 38: 101019.
[24] Xie Y, Xu E, Bowe B, et al. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022; 28(3): 583-590.
[25] Jacobsen PA, Andersen MP, Gislason G, et al. Return to work after COVID-19 infection - A Danish nationwide registry study. Public Health. 2022; 203: 116-122.
[26] Westerlind E., Palstam A., Sunnerhagen K.S., et al. Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Publ Health. 2021; 21(1): 1023.
[27] Watanabe A, Iwagami M, Yasuhara J, et al. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine. 2023; 41(11): 1783-1790.
[28] Zisis SN, Durieux JC, Mouchati C, et al. The Protective Effect of Coronavirus Disease 2019 (COVID-19) vaccination on postacute sequelae of COVID-19: a multicenter study from a large national health research network. Open Forum Infect Dis. 2022; 9: ofac228. 10.1093/ofid/ofac228.
[29] Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022; 22: 43-55.
[30] Cimellaro A, Addesi D, Cavallo M, et al. Monoclonal antibodies and antivirals against SARS-CoV-2 reduce the risk of long COVID: a retrospective propensity score-matched case–control study. Biomedicines 2022, 10, 3135.