Le Infezioni in Medicina, n. 2, 183-201, 2024
doi: 10.53854/liim-3202-7
REVIEWS
Serological and molecular detection of dengue virus in animals: A systematic review and meta-analysis
D. Katterine Bonilla-Aldana1, Marcela María Rodas-Fuenmayor2, Luisa María Ruiz-Aristizabal2, Juan R. Ulloque-Badaracco3, Esteban A. Alarcón-Braga3, Enrique A. Hernandez-Bustamante4,5, Juan C. Cabrera-Guzman3, Ricardo R. Ulloque-Badaracco6, Vicente A. Benites-Zapata7, Alfonso J. Rodriguez-Morales6,8,9
1Research Unit, Universidad Continental, Huancayo, Peru;
2Faculty of Veterinary Medicine, Fundación Universitaria Autónoma de las Américas-Institución Universitaria Visión de las Américas, Pereira, Risaralda, Colombia;
3Escuela de Medicina, Universidad Peruana de Ciencias Aplicadas, Lima, Peru;
4Sociedad Científica de Estudiantes de Medicina de la Universidad Nacional de Trujillo, Trujillo, Peru;
5Grupo Peruano de Investigación Epidemiológica, Unidad para la Generación y Síntesis de Evidencias en Salud, Universidad San Ignacio de Loyola, Lima, Peru;
6Faculty of Health Sciences, Universidad Científica del Sur, Lima, Peru;
7Unidad de Investigación para la Generación y Síntesis de Evidencias en Salud, Vicerrectorado de Investigación, Universidad San Ignacio de Loyola, Lima, Peru;
8Grupo de Investigación Biomedicina, Faculty of Medicine, Fundación Universitaria Autónoma de las Américas-Institución Universitaria Visión de las Américas, Pereira, Risaralda, Colombia;
9Gilbert and Rose-Marie Chagoury School of Medicine, Lebanese American University, Beirut, Lebanon
Article received 4 February 2024, accepted 27 March 2024
Corresponding author
D. Katterine Bonilla-Aldana
E-mail: dbonilla@continental.edu.pe
SummaRY
Introduction: Dengue is a vector-borne disease, especially important in tropical and subtropical areas. The first presentation of many arboviral diseases occurred mainly in animals, including multiple Alphaviruses and Flaviviruses, such as dengue.
Objective: To determine the serological and molecular frequency of the dengue virus in animals.
Methods: A systematic literature review was carried out in five databases for the proportion of animals infected with dengue, defined by molecular and serological tests. A meta-analysis was performed using a random-effects model to calculate the pooled prevalence and 95% confidence intervals (CI). Cochran?s Q test and the I2 statistic were used to assess the heterogeneity between the two studies.
Results: The presence of dengue in bats, primates, birds, sheep, horses, cattle, pigs, rodents and buffaloes, according to serological methods, had a prevalence of 10%, 29%, 8%, 1%, 11%, 0%, 49%, 2%, 7%, respectively. According to molecular methods, the presence of dengue in bats had a seroprevalence of 6.0%.
Conclusion: The present study confirms the presence of the Dengue virus in a large group of animal species, with potential implications as possible reservoirs of this virus, raising the possibility of zoonotic transmission.
Keywords: Animals, dengue, virus, zoonosis, systematic review, and meta-analysis.
INTRODUCTION
Dengue continues to be a significant public health threat and the most important arboviral disease in terms of morbidity and mortality globally [1, 2]. The Dengue virus (DENV) corresponds to the Dengue serocomplex, Flavivirus genus, Flaviviridae family. There are four distinct but closely related serotypes: DENV1, DENV2, DENV3, and DENV4; these periodically cross endemic and hyperendemic areas, and all cause the disease known as Dengue [3].
Each of the four Dengue serotypes generates a unique immune response to infection in the host. They are distributed throughout tropical and subtropical regions worldwide. The four serotypes are genetically similar, share approximately 65% of their genome, and are transmitted to non-human primates (jungle form) and humans (human form), mainly by the Aedes genus mosquito [4].
The main vectors of the DENV are the Aedes aegypti, Aedes albopictus, and Aedes vittatus mosquitoes, which have been recently reported in the Americas [5]. They live in urban habitats where they reproduce in containers with accumulated water, and their feeding is during the day; the periods of the bites are intensified in the early morning and the evening before it gets dark. The cycle comprises four stages: egg, larva, pupa, and adult, lasting 8 to 10 days [6].
Transmission of the virus occurs mainly through the bite of infected female mosquitoes. After biting the host, the mosquito expels saliva filled with the virus into the human?s blood. The incubation period for the virus lasts between 4 and 10 days, and an infected mosquito can transmit the pathogen for a lifetime. Asymptomatic and symptomatic infected humans are the primary carriers of the virus, and mosquitoes become infected by biting them. After the appearance of the first symptoms, these infected people can transmit the infection for 4 or 5 days, 12 days maximum, to Aedes mosquitoes [7]. In addition to the urban cycle, there is at least one jungle cycle in which other vectors can potentially participate, where animals can play an important role [8].
Some studies have identified DENV in animals; this is the case of a study in which 293 equine serum samples were analysed in the French Pacific Islands, where they found 7.7% positivity for Dengue virus serotype 1 (DENV1) [9]. In Thailand, in a study of 38 macaques (Macaca nemestrina), it was determined by serology that 24% were positive for DENV [10]. In another study carried out in Costa Rica and Ecuador, neutralizing antibodies to serotypes 1 and 2 (22.6%) and 3 (30.0%) of the Dengue virus were detected in bats [11]. Finally, in another study in Colombia, in the departments of Córdoba and Sucre, they sampled 286 non-hematophagous bats, Carollia perspicillata and Phyllostomus decoloran, where they found that the amplicons showed a high similarity with Dengue virus serotype-2 (DENV-2), being the first evidence of the DENV-2 genome in bats from the Colombian Caribbean [12].
Concerning the clinical findings in animals infected with dengue, there are no appropriate animal models for the study of the physio-pathogenesis and the clinical manifestations of the disease, nor there are studies as references for the evaluation of specific pharmacological treatment. No animal suffers clinical manifestations similar to those expressed by humans, whether infected by mosquitoes or experimentally [8].
DENV is a severe disease with epidemiological, social, and economic impact, which has become a growing problem for global public health. In addition, it is an emerging and re-emerging disease of greater magnitude and importance due to its tremendous economic impact on the exposed population. Therefore, it represents a severe public health problem in the American Region [13].
The disease in humans can be asymptomatic or present symptoms that vary from mild fever to impatient fever, accompanied by severe headache, pain behind the eyes, pain in muscles and joints, and erythema; it can also progress to severe forms, mainly characterized by shock, respiratory distress and severe organ damage [14].
In most tropical Americas, Dengue epidemics occur periodically in different countries, especially Brazil, Colombia, and Venezuela [15]. The four virus serotypes are present in America, and their co-circulation was reported in Brazil, Guatemala, and Mexico. This simultaneous circulation of two or more serotypes increases the occurrence of severe disease cases. Due to its population size, Brazil has the highest number of registered cases, followed by Mexico, Nicaragua, and Colombia, with 106,066 cases reported in 2019 [10]. Diagnostic methods for the detection of DENV in humans include RT-PCR (Reverse transcriptase-polymerase chain reaction), ELISA (Enzyme-Linked Immunoassay), nonstructural protein one antigen (NS1 antigen), hemagglutination inhibition (HI), and immunochromatography [15].
Demographic, social, and environmental factors, such as unplanned urbanization, migration, cultural aspects, housing conditions, and the quality of health service provision, have influenced the spread of the vector, increasing the incidence and appearance of the disease in new geographical areas.
Climate change influences the DENV, as an increase of 1 to 2 degrees of temperature extends the development of the vectors, which are poikilothermic. This study aims to determine the serological and molecular frequency of the Dengue virus in animals, according to species, countries, and serotypes, and to analyse the average viral load of the virus in animal reservoirs.
METHODS
Registration and reporting
A summarised version of the protocol for this systematic review was uploaded to the International Prospective Register of Systematic Reviews (PROSPERO), and we drafted our results using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16].
Search strategy and databases
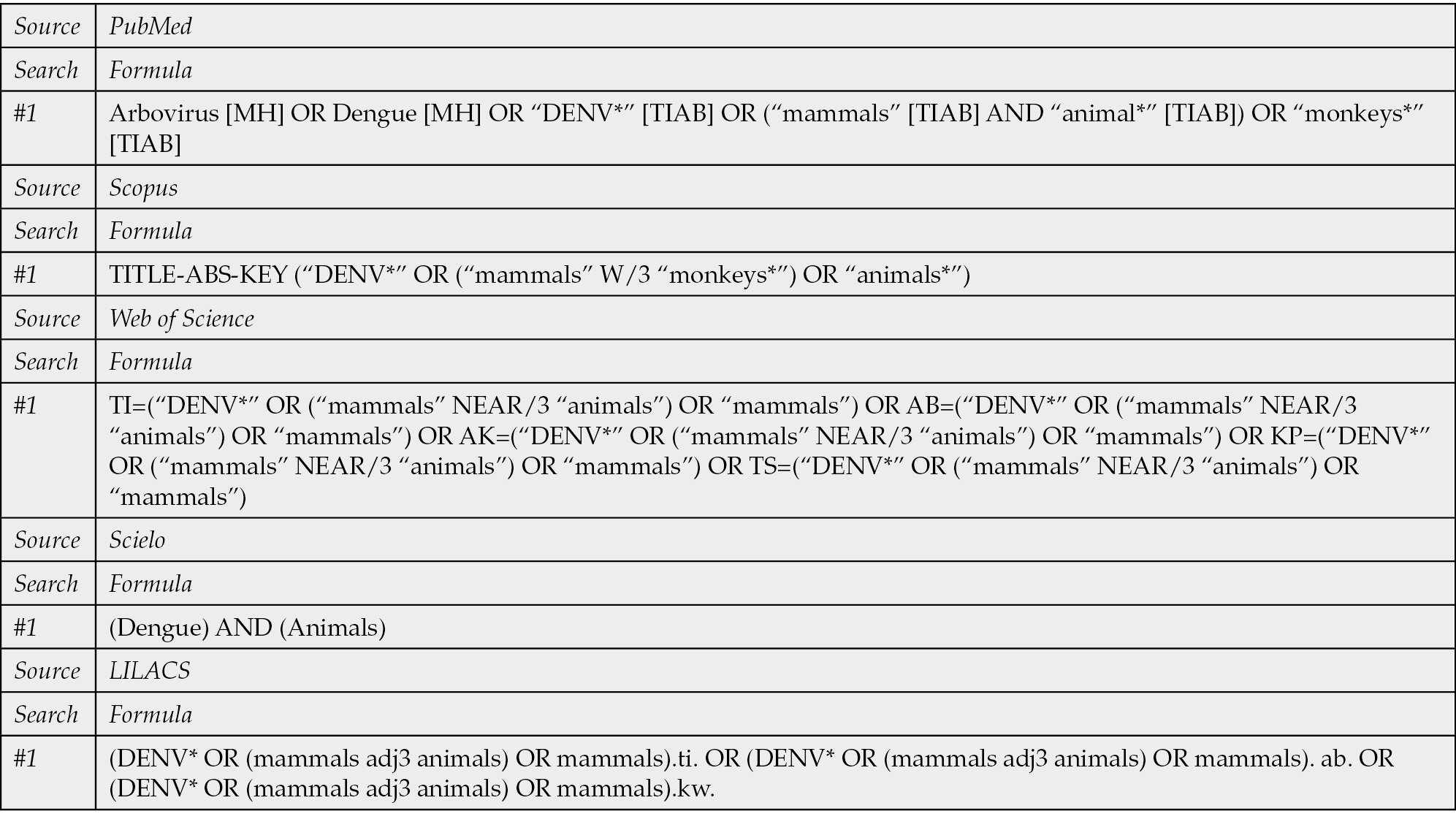
The search strategy followed the Peer Review of Electronic Search Strategies (PRESS) Checklist [17]. The search terms were based on MeSH and accessible terms for “Dengue animals”, “Dengue reservoirs”, “Prevalence Dengue animals”, and “Prevalence Dengue reservoirs”. Afterwards, the search formula was adapted for all databases without language restrictions. We run the systematic search (March 10, 2023) through the following databases: PubMed, Scopus, Web of Sciences, LILACS and Scielo. We detailed the complete search strategy in Table 1.
Table 1 - Search strategies.
Study selection and data extraction
We performed each phase of the study selection process independently and by at least two authors. The eligibility criteria were:
- observational studies reporting the
- infection by DENV in animals with serological or molecular tests.
ELISA, microneutralisation test (MNT), plaque reduction neutralisation test (PRNT), HI, complement fixation (CF), and NS1 antigen were considered for serological tests, and RT-PCR for tests based on molecular biology. We excluded narrative reviews, scoping reviews, systematic reviews and conference abstracts. References with incomplete information were also excluded. We removed duplicates with Rayyan QCRI © [18]. Two authors screened the remaining references by titles and abstracts in Rayyan. The authors independently assessed the full text of the relevant references to be included. We resolved any conflict or discrepancy in any phase of the study selection process by consensus. Two authors independently extracted data using a standardized data extraction sheet built-in Google Sheets ©. The following information was extracted: author, publication type, publication date, publishing institution, country, sample size, infected animals, method of detection, and serological or molecular tests.
Risk of bias and publication bias
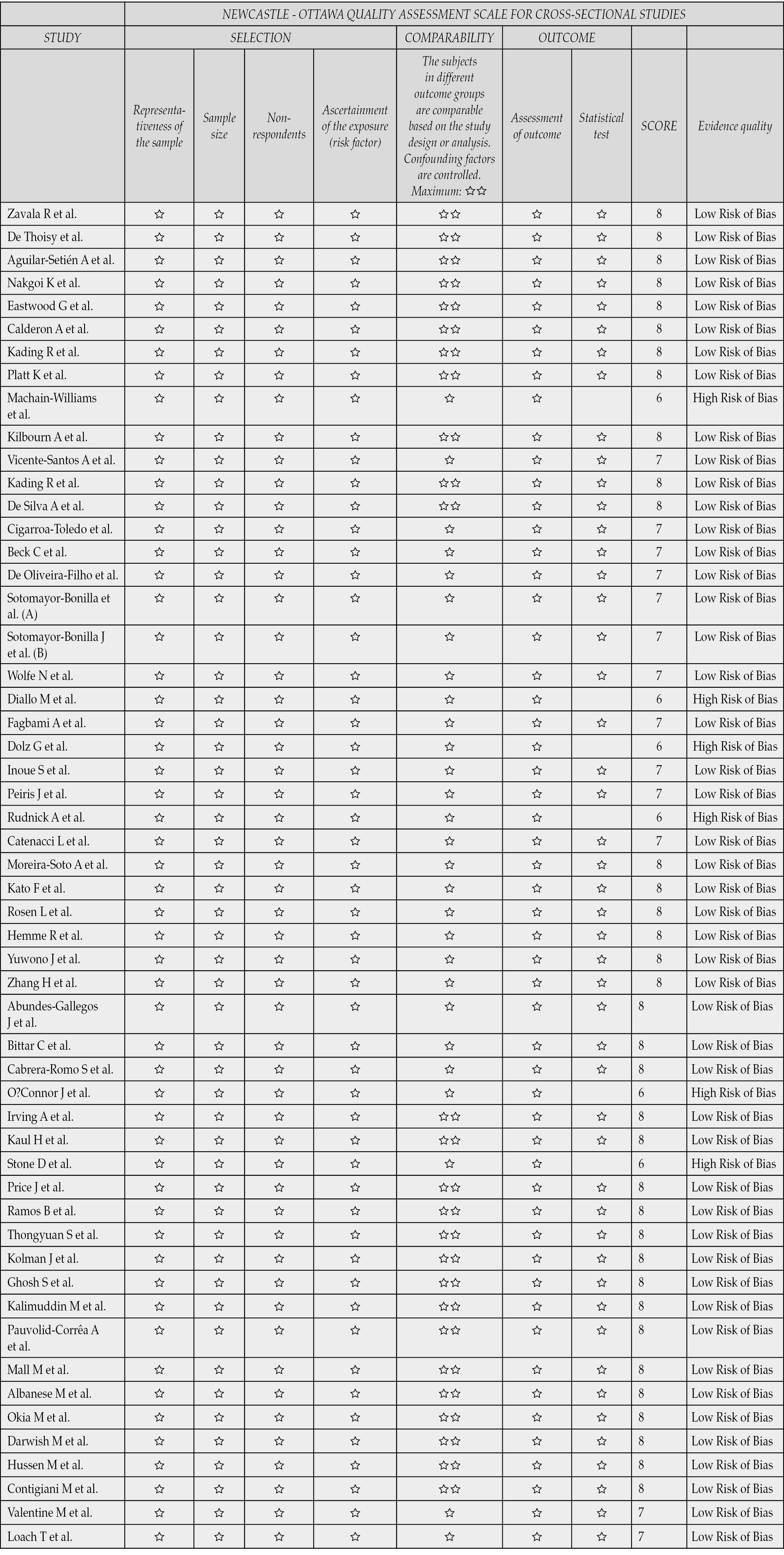
The quality assessment process was performed independently by two authors. We used the adapted version of the Newcastle-Ottawa Scale for cross-sectional studies (NOS-CS). A score ≥7 stars was considered a low risk of bias; otherwise, the study was considered to have a high risk of bias [19]. The publication bias assessment was not performed because it is not recommended for proportional meta-analysis. After all,
- conventional funnel plots and Egger?s test are inaccurate for these analyses, and
- there is no evidence that proportions adjust correctly to funnel plots or Egger?s tests [20, 21].
Data analysis
STATA 17.0 © was used for performing statistical analysis. We conducted a pooled analysis of the prevalence of DENV-infected animals according to serological or molecular tests. The 95% Confidence Intervals (CI) for the proportions reported in each study were calculated using the Clopper-Pearson Method. The Freeman-Tukey Double Arcsine Transformation was used as the variance stabiliser. We used a random-effects model (Dersimonian and Laird) for the quantitative analysis. We assessed the between-study heterogeneity using Cochran?s Q test and the I2 statistic. Values equal to or greater than 60% were defined as high heterogeneity for the I2 statistic, and p-values <0.05 are considered indicators of heterogeneity in Cochran?s Q test. In addition, we carried out subgroup analysis using the serological method and continents. Sensitivity analyses were performed using only studies with a low risk of bias.
RESULTS
Selection and characteristics of the studies
The systematic search retrieved 3921 records; after removing duplicates, 2352 records remained. After excluding articles by title and abstract and assessing their full-text documents, 54 studies were identified as eligible for the qualitative and quantitative syntheses [9-12, 22-71]. The flow diagram summarizing the study retrieval is shown in Figure 1.
The characteristics of the studies are presented in Table 2. The included studies were conducted between 1958 and 2020 with 11824 animals. Regarding evaluating the quality of the studies with the NOS, 48 were at a low risk of bias, and the remaining six were at a high risk of bias (Table 3).
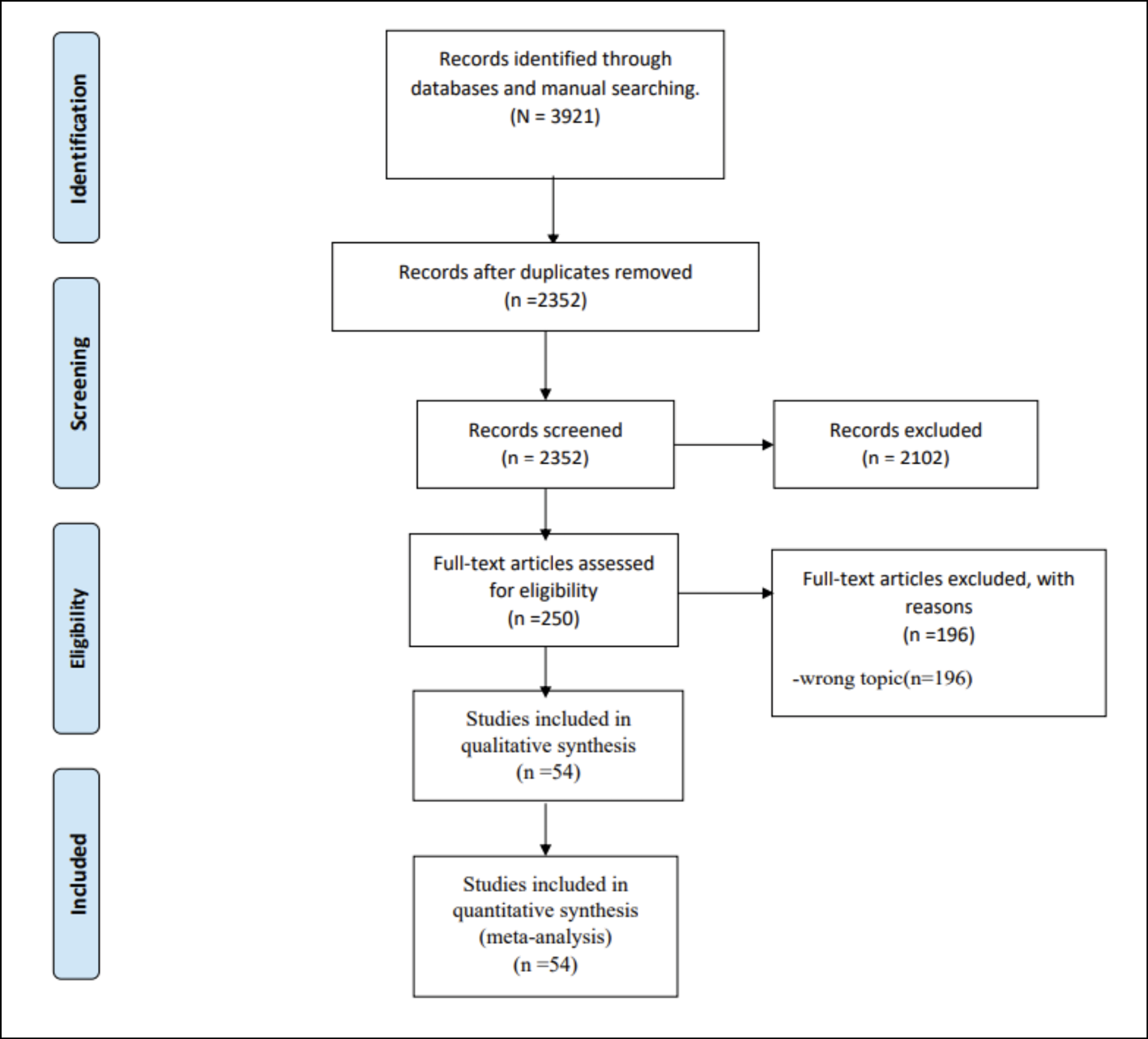
Figure 1 - PRISMA Flow Diagram.
Table 2 - Characteristics of the included studies.
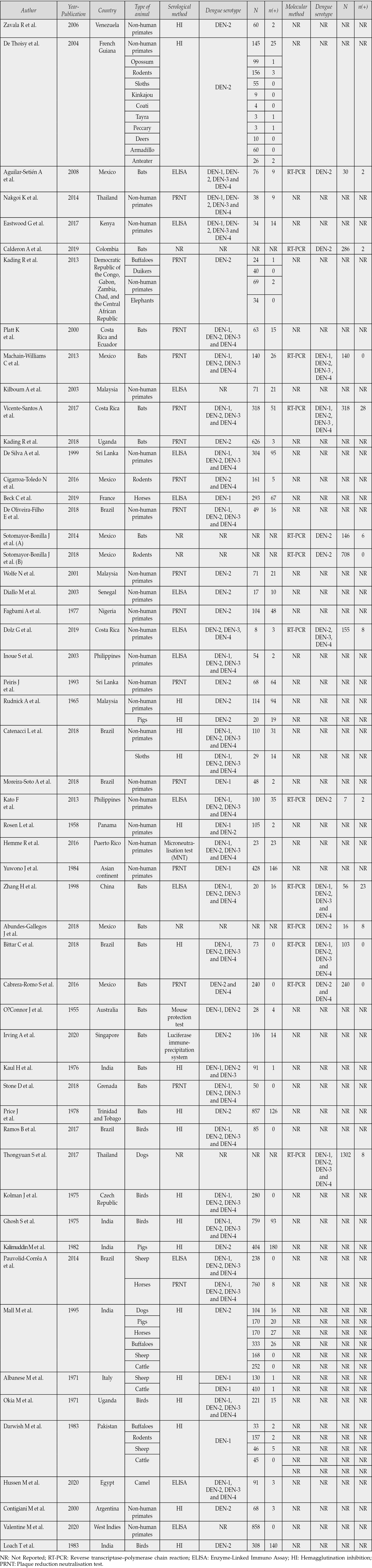
Table 3 - Quality assessment of included studies.
Bats
Serological methods
The presence of dengue in bats according to serological methods was evaluated in 13 studies (n=2688), with a seroprevalence of 10.0% (95% CI: 4.0%-17.0%; I2=96.62%) (Figure 2). When subgroup analysis was performed according to the type of serological methods (Figure 3), it was found that bats evaluated by PRNT, ELISA and HI had a prevalence of 6.0%, 23.0%, and 3.0%, respectively. In the subgroup analysis according to continents, it was found that the bats evaluated in America and Asia had a prevalence of 8.0% and 24.0%, respectively (Figure 4). In their sensitivity analysis, no decrease in their heterogeneity was found, with a prevalence of 10.0% (95% CI: 3.0%-19.0%; I2=97.25%) (Figure 5).
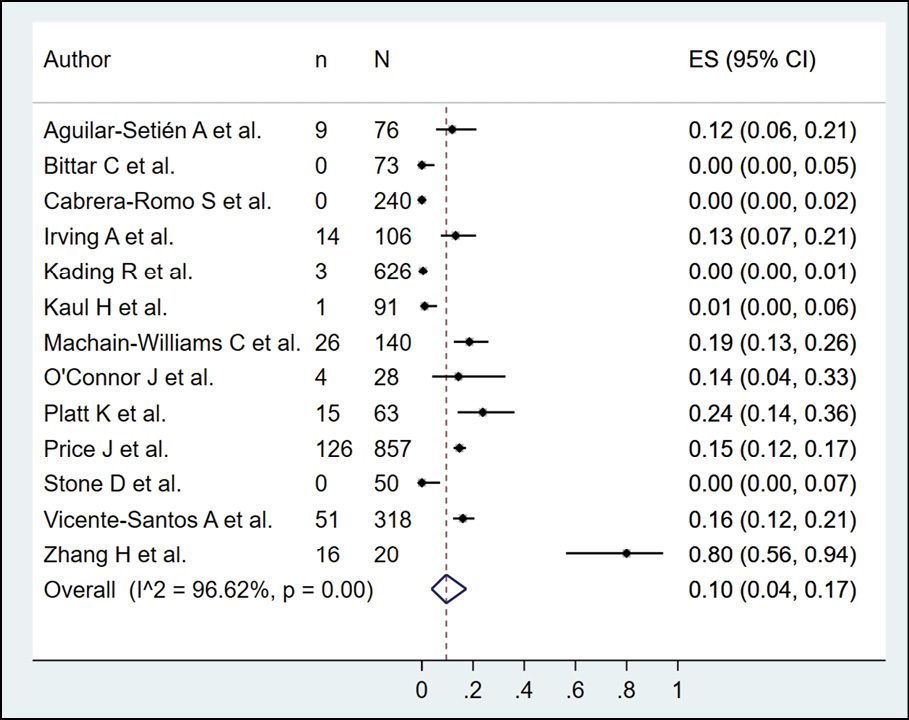
Figure 2 - Prevalence of dengue in bats according to serological method.
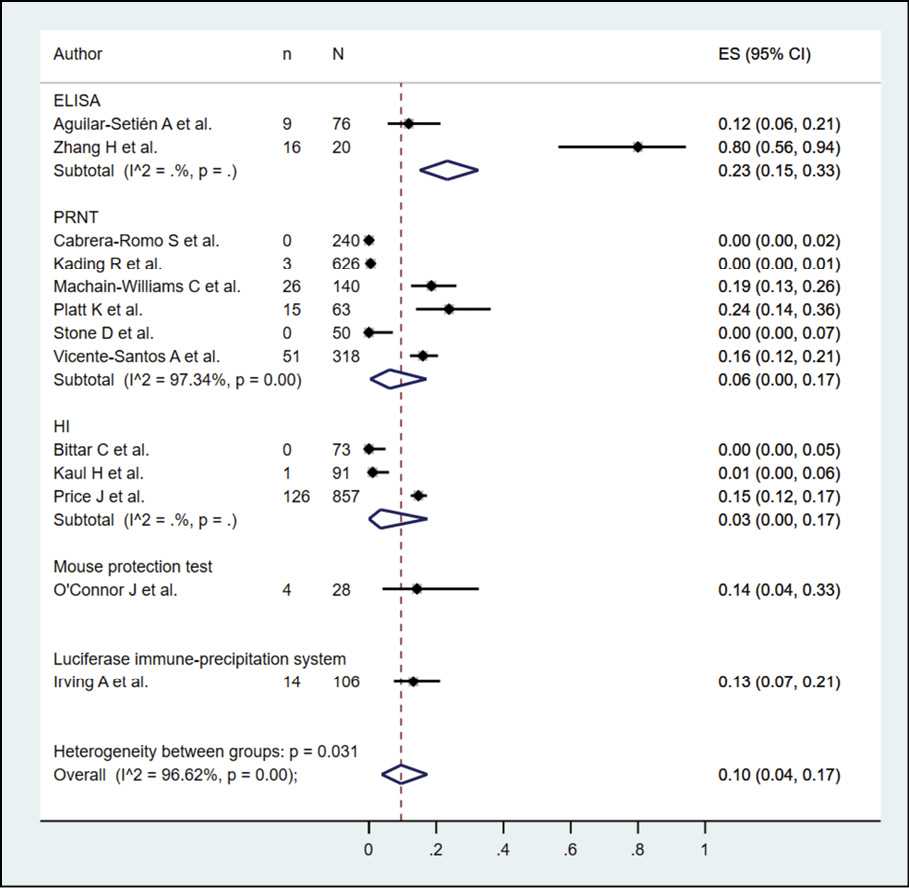
Figure 3 - Subgroup analysis according to type of serological method in the prevalence of dengue in bats.
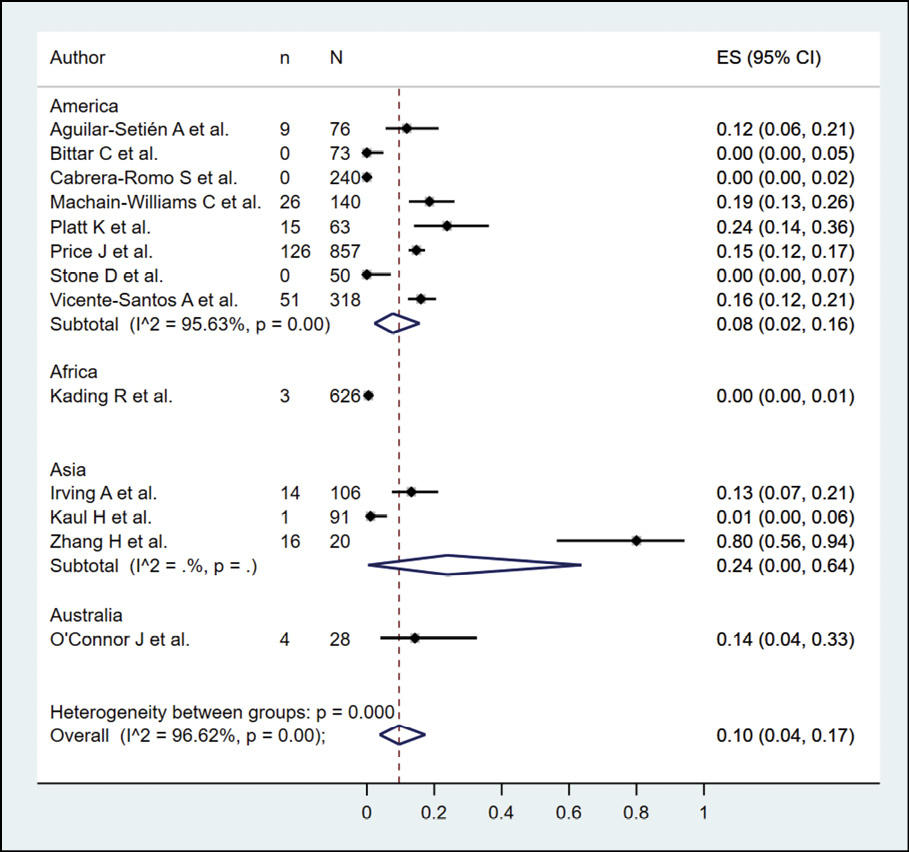
Figure 4 - Subgroup analysis according to continents in the prevalence of dengue in bats.
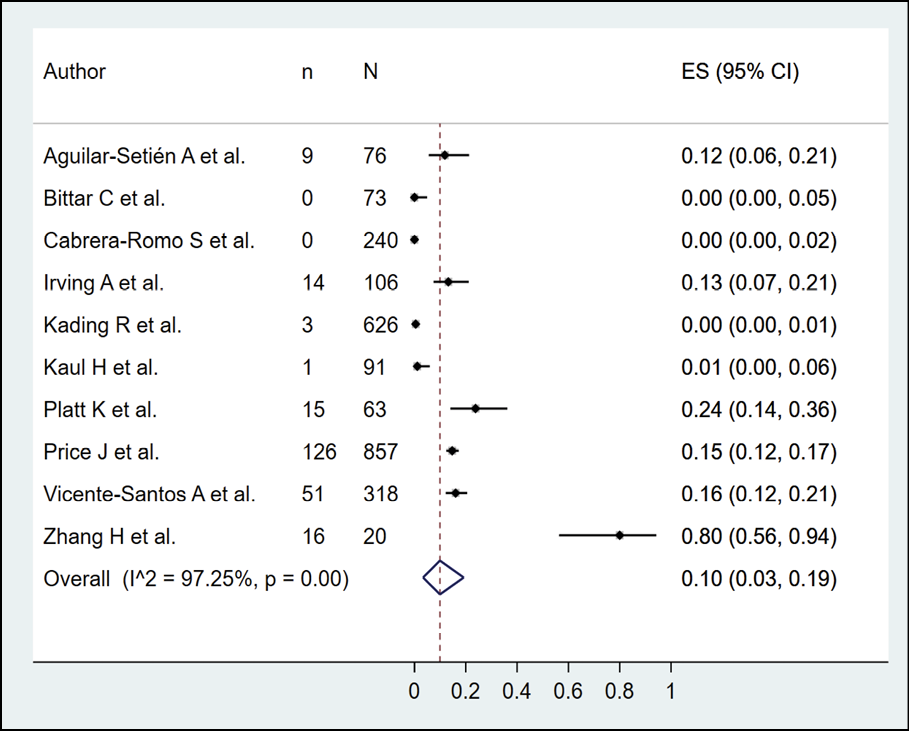
Figure 5 - Sensitivity analysis according to the risk of bias in the prevalence of dengue in bats.
Molecular methods
The presence of dengue in bats according to molecular methods (RT-PCR) was evaluated in 9 studies (n=1335), with a seroprevalence of 6.0% (95% CI: 1.0%-13.0%; I2=94.78%) (Figure 6).
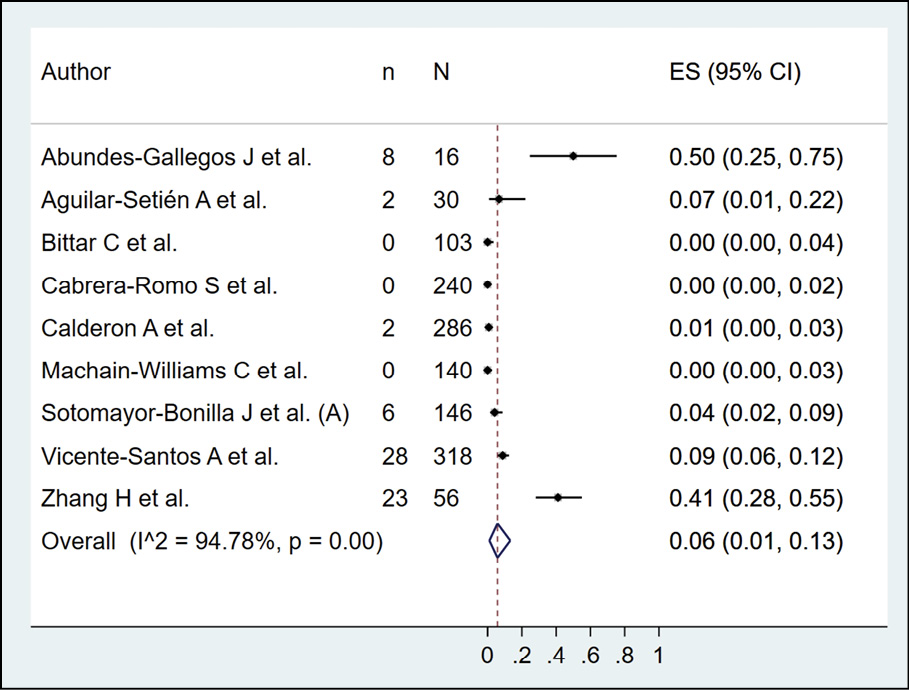
Figure 6 - Prevalence of dengue in bats according to molecular method.
Non-human primates (NHP)
Serological methods
The presence of dengue in NHP according to serological methods was evaluated in 23 studies (n=2946), with a seroprevalence of 29.0% (95% CI: 16.0%-44.0%; I2=98.46%) (Figure 7). When subgroup analysis was performed according to the type of serological methods (Figure 8), it was found that NHP evaluated by PRNT, ELISA and HI had a prevalence of 32.0%, 25.0% and 19.0%. In the subgroup analysis, according to continents (Figure 9), it was found that the bats evaluated in America, Asia, and Africa had a prevalence of 18.0%, 40.0%, and 33.0%, respectively. In their sensitivity analysis, no decrease in their heterogeneity was found, with a prevalence of 25.0% (95% CI: 13.0%-39.0%; I2=98.37%) (Figure 10).
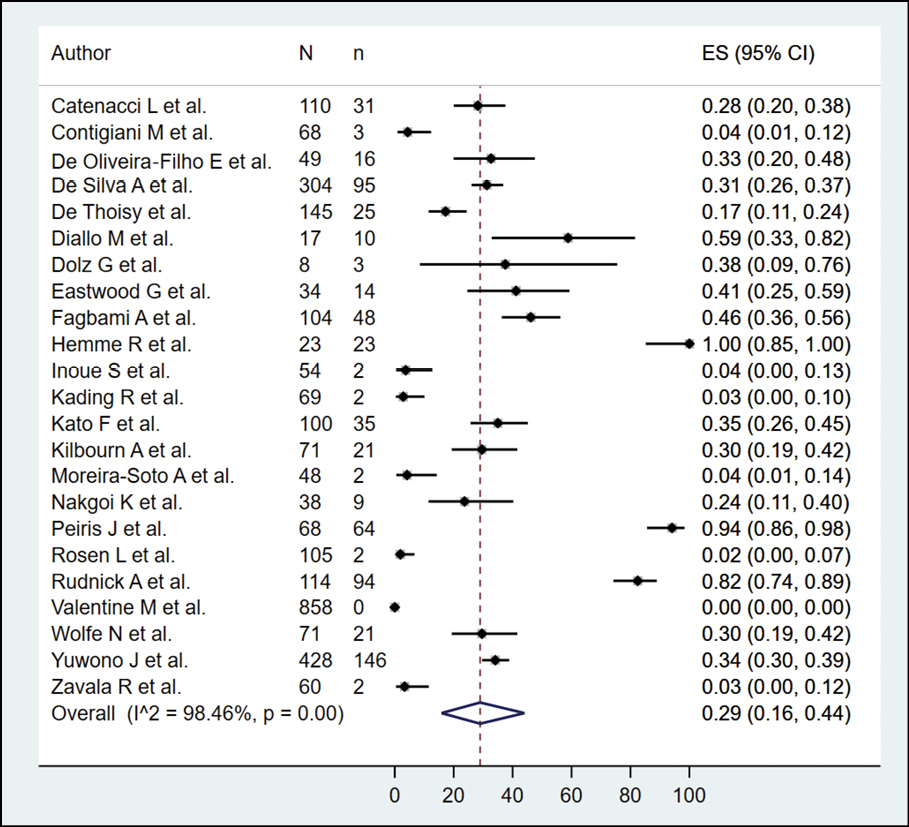
Figure 7 - Prevalence of dengue in non-human primates according to serological method
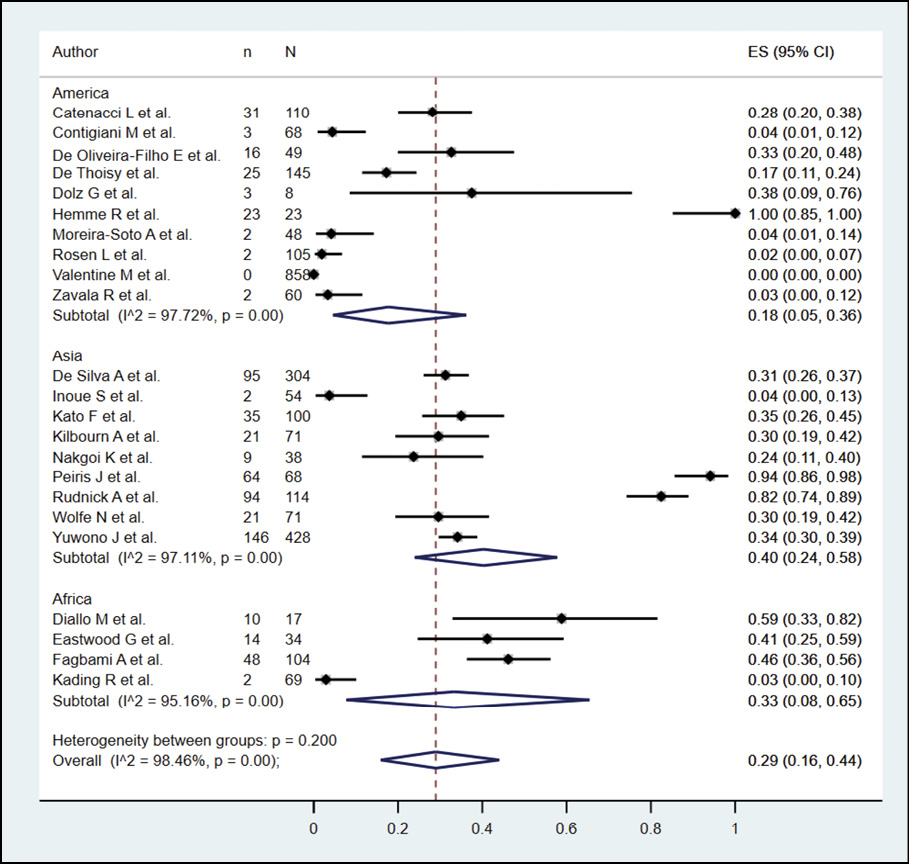
Figure 8 - Subgroup analysis according to type of serological method in the prevalence of NHP in bats.
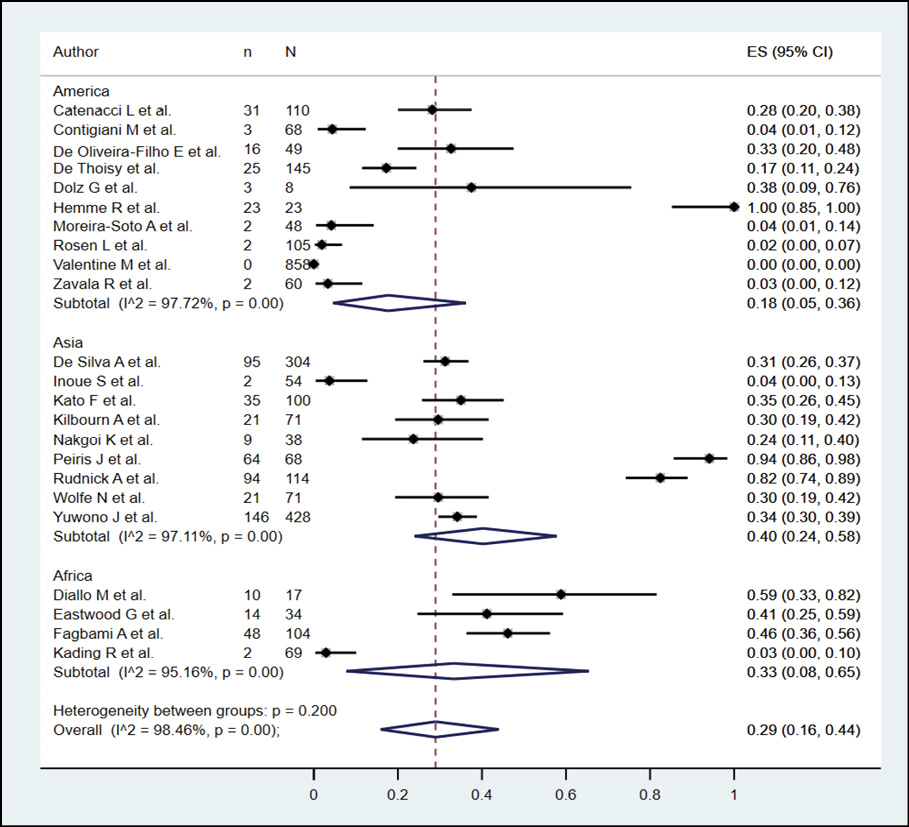
Figure 9 - Subgroup analysis according to continents in the prevalence of dengue in NHP.
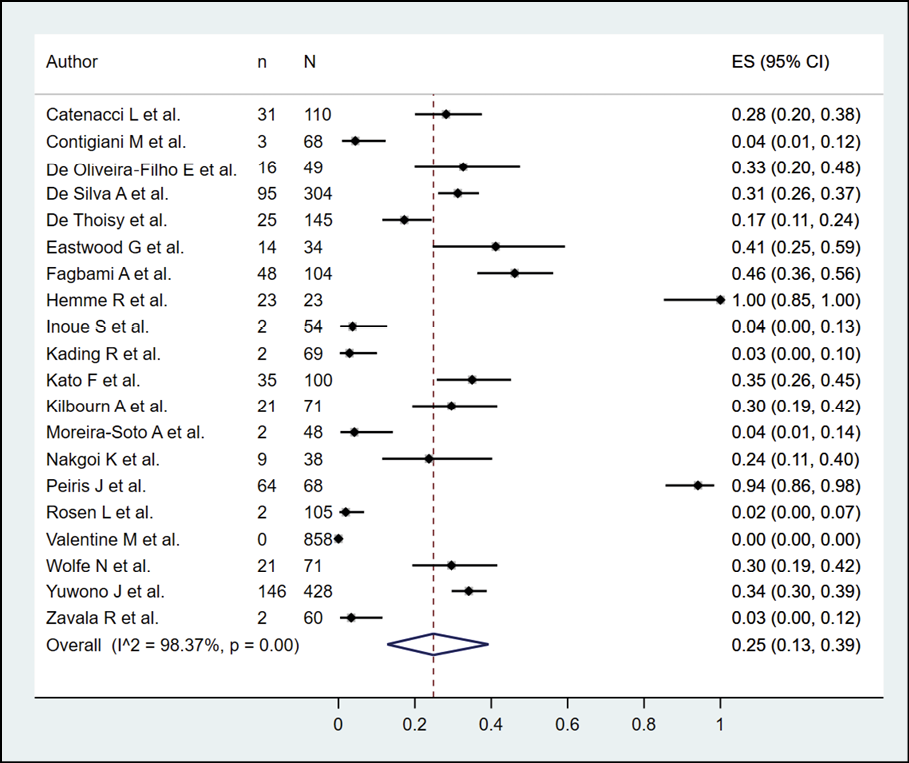
Figure 10 - Sensitivity analysis according to the risk of bias in the prevalence of dengue in NHP.
Other animals
The presence of dengue in birds according to serological methods was evaluated in 5 studies (n=1653), with a seroprevalence of 8.0% (95% CI: 0.0%-25.0%; I2=98.82%) (Figure 11). The occurrence of dengue in sheep by serological methods was evaluated in 4 studies (n=582), with a seroprevalence of 1.0% (95% CI: 0.0%-4.0%; I2=82.42%) (Figure 12). In the case of horses, dengue was assessed by serological methods in 3 studies (n=1223), with a seroprevalence of 11.0% (95% CI: 0.0%-33.0%; I2=98.7%) (Figure 13).
The presence of dengue in cattle by serological methods was evaluated in 3 studies (n=707), with a seroprevalence of 0.0% (Figure 14). The detection of dengue in pigs by serological methods was evaluated in 3 studies (n=594), with a seroprevalence of 49.0% (95% CI: 16.0%-83.0%; I2=98.1%) (Figure 15). The identification of dengue in rodents by serological methods was evaluated in 3 studies (n=474), with a seroprevalence of 2.0% (95% CI: 1.0%-4.0%; I2=98.1%) (Figure 16). Finally, the occurrence of dengue in buffaloes by serological methods was evaluated in 3 studies (n=390), with a seroprevalence of 7.0% (95% CI: 4.0%-10.0%; I2=0%) (Figure 17).
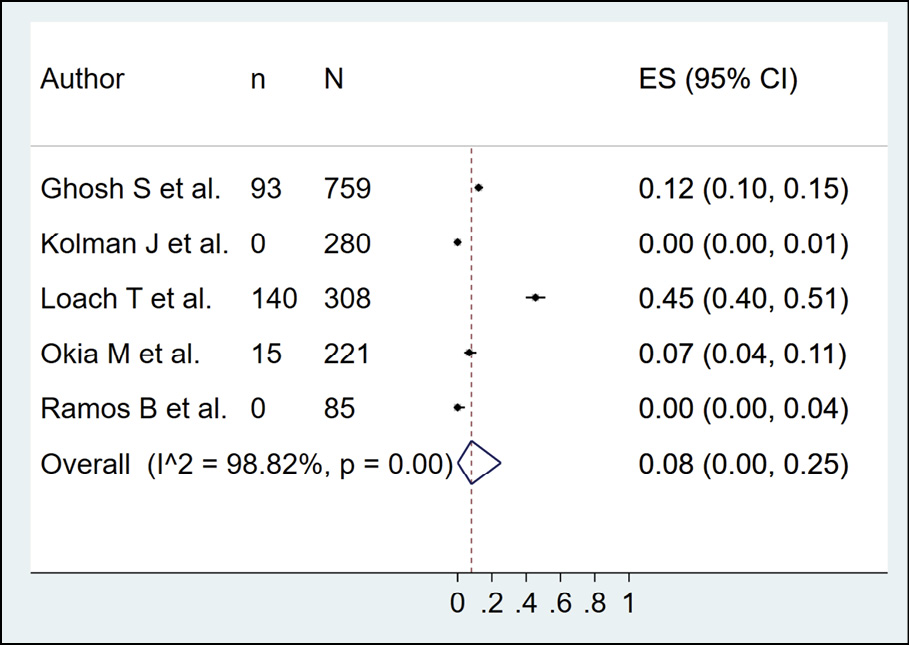
Figure 11 - Prevalence of dengue in birds according to serological method.
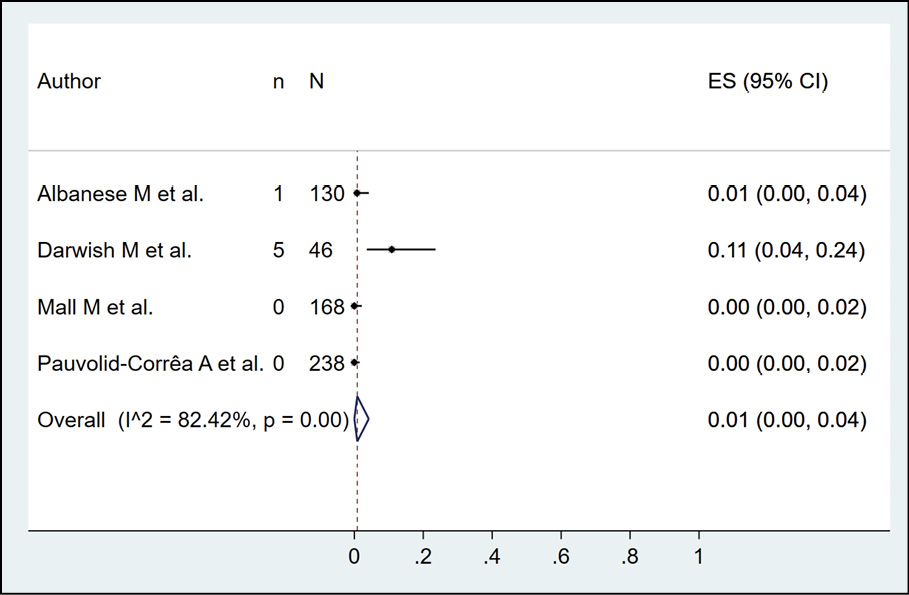
Figure 12 - Prevalence of dengue in sheeps according to serological method.
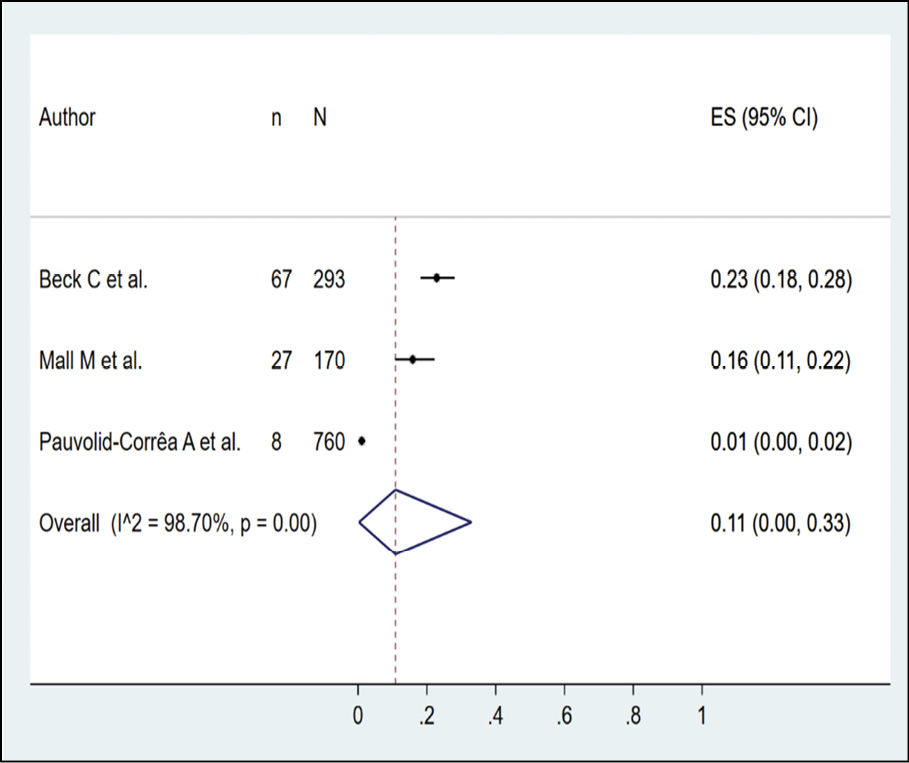
Figure 13 - Prevalence of dengue in horses according to serological method
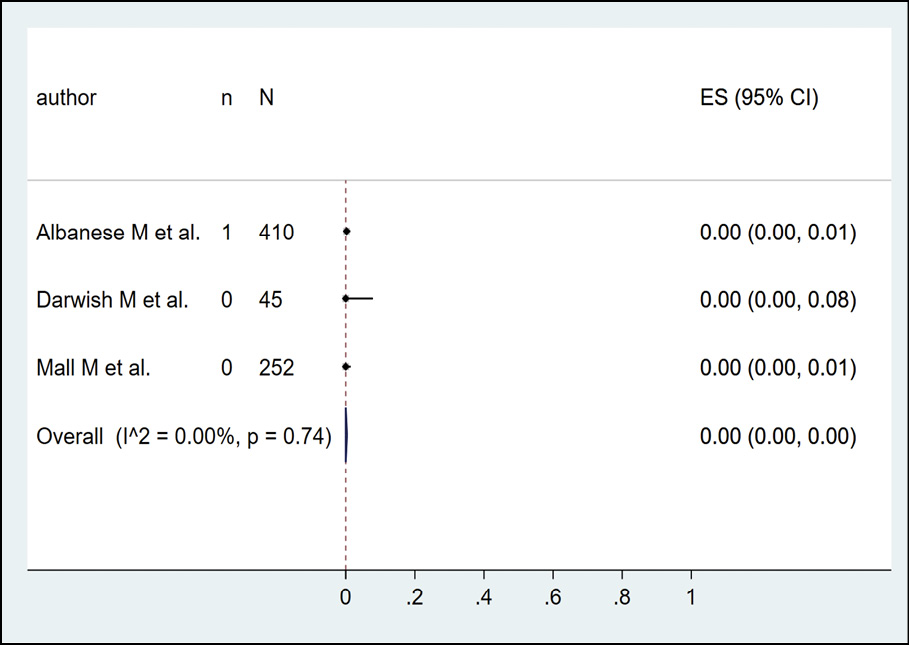
Figure 14 - Prevalence of dengue in cattle according to serological method
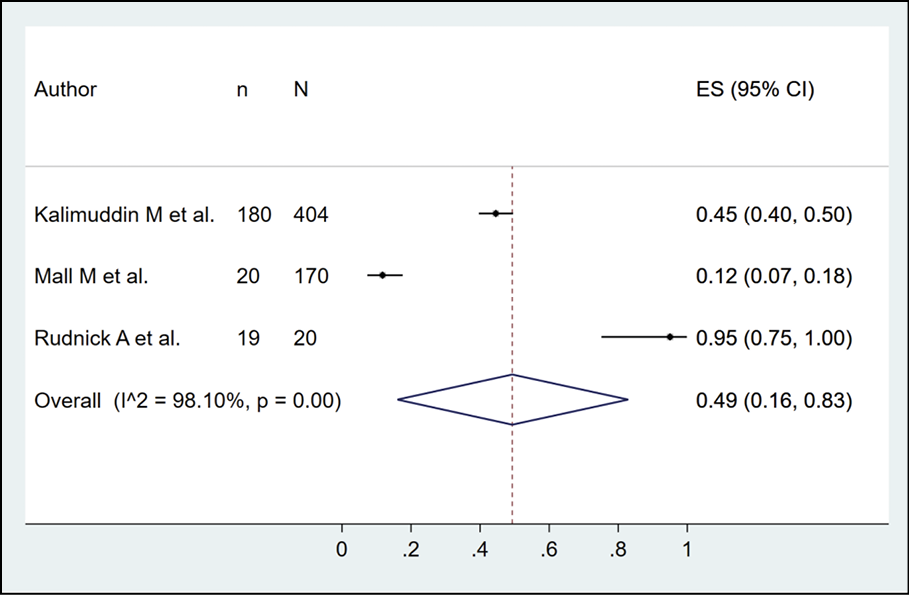
Figure 15 - Prevalence of dengue in pigs according to serological method.
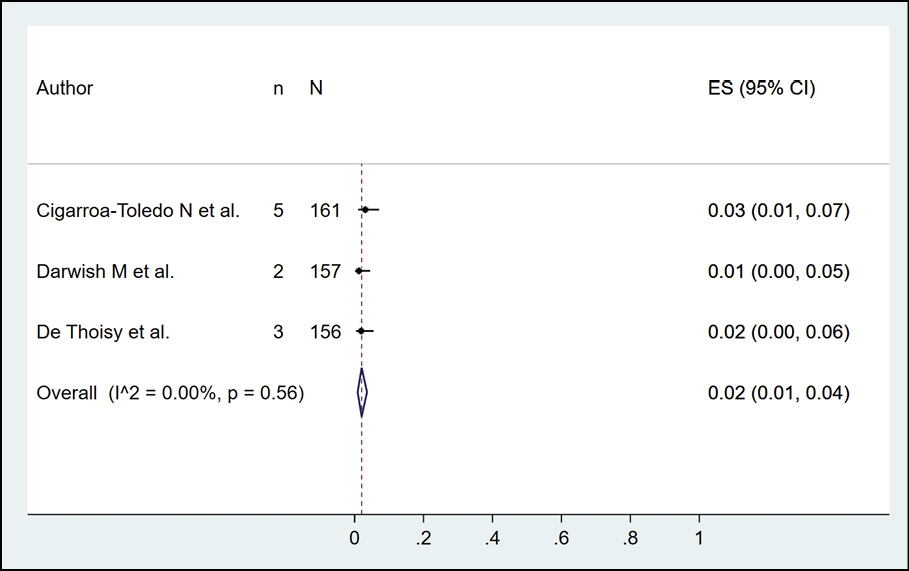
Figure 16 - Prevalence of dengue in rodents according to serological method.
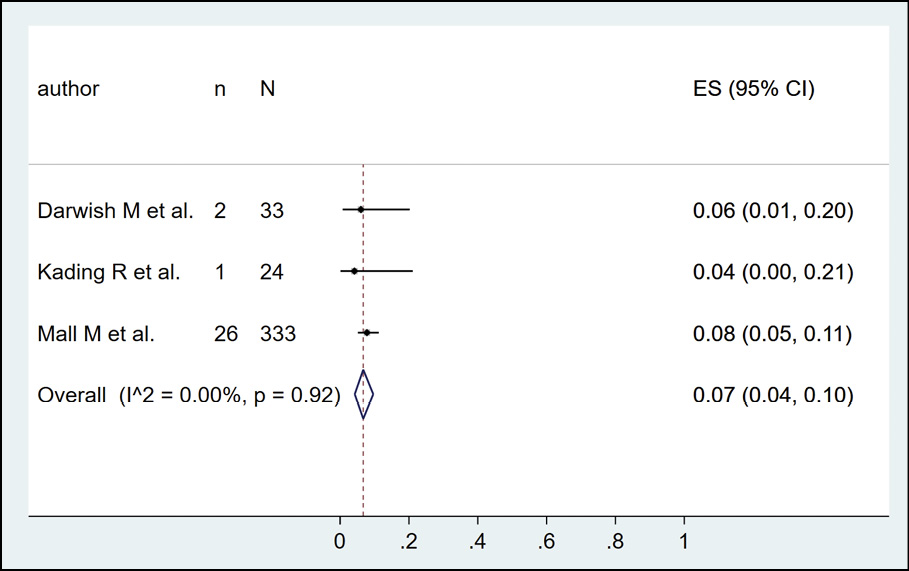
Figure 17 - Prevalence of dengue in buffaloes according to serological method
DISCUSSION
During the last few years, an essential part of the research related to Arboviruses, such as dengue, has focused on better understanding the factors associated with its transmission, including understanding the animal reservoirs that could potentially serve as a natural source in the environment, from which transmission cycles can be established in wild and suburban areas, but perhaps also urban ones, using different urban and wild vector insects, of which Aedes aegypti, Aedes albopictus. Aedes vittatus has also stood out, recently reported on the American continent. Dengue involves the sylvatic (enzootic) cycle and the endemic urban cycle, which involve non-human primates in sylvan habitats and humans in urban settings as reservoir hosts [72]. However, based on existing studies, not all primate species may be susceptible to Dengue virus infection [73].
Apart from non-human primates, bats are the most studied in detecting the presence of Dengue infection, and most studies include RT-PCR. As recently described in Colombia, in the department of Córdoba, with Carollia perspicillata and Phyllostomus discolor, two studies have confirmed the presence of Dengue virus in these bats [74]. In these studies, serotype 2 (DENV-2) has been identified with molecular diagnosis and sequencing.
In addition, other studies in Latin America, such as Mexico, found six bats (4.1%) positive for DENV-2 [75]. On the other hand, in Costa Rica and Ecuador, neutralising antibodies to dengue virus serotypes 1 and 2 and serotypes 2 and 3 were detected in 12 of 53 (22.6%) and 3 of 10 (30.0%) bats, suggesting that bats can be infected with the Dengue virus.
Bats are evolutionarily successful creatures widely distributed globally [76]. It is known that at least one species from each of the 19 families that make up the order Chiroptera perches in dwellings, which has implications for the bat-human closeness, which the presence of vector insects also surrounds, such as Aedes, which are not only anthropophilic but simultaneously zoophilic (amphiphilic) [77, 78].
In a previous systematic review covering up top 2019, Dengue positivity was detected in bats (10.1%), non-human primates (NHP) (27.3%), while in our study, 10.0% was also found in bats by serology, but was 24% in Asia, with 6% by molecular methods [77]. For NHP the seroprevalence was 29%, reaching 40% in Africa.
Relatively high DENV seroprevalence was also observed in marsupials, the primary reservoirs of the Ross River virus, a mosquito-borne Alphavirus [79]. In addition, dengue positivity was observed in birds, dogs, and rodents, animals commonly found in the urban environment. Based on the available evidence, it is not recommended to isolate animals in a general way. Phylogenetic studies would be interesting during epidemics, assessing the strains of dengue circulating in humans and animals, which may suggest transmission between them.
By extension, the abundance of these animals in urban settings potentially translates into a possible increased risk of exposure to dengue in humans who also become infected through mosquito bites. In addition, studies have also observed a wide range of hosts feeding on vector mosquitoes that efficiently transmit dengue, namely Aedes albopictus and Aedes aegypti and even now Aedes vittatus, present in the American continent [5, 80, 81].
The Dengue virus continues to represent a complex global public health problem, even worse in times of COVID-19 due to its cocirculation and coinfections, and in which a comprehensive, holistic vision of health is always required, where the role of the environment and animal health, can play a critical role. Veterinary research is needed to realise the importance of this last component [82]. In addition, they have begun to show the virus?s relationships and presence in these animals. Finally, however, it is better to understand its weight in the different transmission cycles.
CONCLUSIONS
Several studies have shown that nucleic acids or antibodies to Dengue Virus (DENV) are present in Neotropical wildlife, including bats, suggesting that some species may be susceptible to DENV infection. Non-human primates have been widely used as models for studies on the pathogenesis of dengue and therapeutic interventions. However, they are also animals where the virus has been detected in natural conditions. Dengue virus can infect several animal species; however, its role as an amplifying reservoir is uncertain due to several limitations in the evidence. That suggests the need for more studies. In addition, the results lead to a greater need for more studies to evaluate the role in transmission, including assessing the feeding preference of the vectors. Given the relevant proportion of animals with positive results for dengue, surveillance in animals, especially during epidemics in humans, would be interesting, as in those endemic areas, as well as to increase surveillance in vectors, including studies of host preferences by Aedes species. Finally, they imply a greater need for studies in Colombia, an endemic disease country.
Conflict of interest
None.
Funding
Support from Universidad Continental was received to cover the Article Processing Charges of this publication.
Ethical approval statement
Not applicable.
REFERENCES
[1] Musso D, Rodriguez-Morales AJ, Levi JE, Cao-Lormeau VM, Gubler DJ. Unexpected outbreaks of arbovirus infections: lessons learned from the Pacific and tropical America. The Lancet. Infect Dis. 2018; 18: e355-e361.
[2] Rodriguez-Morales AJ, Villamil-Gomez WE, Franco-Paredes C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med Infect Dis. 2016; 14: 177-179.
[3] Velandia ML, Castellanos JE. Virus del dengue: estructura y ciclo viral. Infectio. 2011; 15: 33-43.
[4] Arredondo-García JL, Méndez-Herrera A, Medina-Cortina H. Arbovirus en Latinoamérica. Acta Pediatr Mex. 2016; 37: 111-131.
[5] Alarcón-Elbal PM, Rodríguez-Sosa MA, Newman BC, Sutton WB. The First Record of Aedes vittatus (Diptera: Culicidae) in the Dominican Republic: public health implications of a potential invasive mosquito species in the Americas. J Med Entomol. 2020; 57: 2016-2021.
[6] Luo H, Winkelmann ER, Fernandez-Salas I, et al. Zika, dengue and yellow fever viruses induce differential anti-viral immune responses in human monocytic and first trimester trophoblast cells. Antiviral Res. 2018; 151: 55-62.
[7] Hasty JM, Felix GE, Amador M, et al. Entomological Investigation Detects Dengue Virus Type 1 in Aedes (Stegomyia) albopictus (Skuse) during the 2015-16 Outbreak in Hawaii. Am J Trop Med Hyg. 2020; 102: 869-875.
[8] Burgos Sojosa BY, Loaiza Montalvo GD, Solórzano Gorozabel MS, Vásconez Moreno LG. Fisiopatología del dengue. RECIMUNDO. 2019;3.
[9] Beck C, Leparc-Goffart I, Desoutter D, et al. Serological evidence of infection with dengue and Zika viruses in horses on French Pacific Islands. PLoS Negl Trop Dis. 2019; 13:e0007162.
[10] Nakgoi K, Nitatpattana N, Wajjwalku W, et al. Dengue, Japanese encephalitis and Chikungunya virus antibody prevalence among captive monkey (Macaca nemestrina) colonies of Northern Thailand. Am J Primatol. 2014; 76: 97-102.
[11] Platt KB, Mangiafico JA, Rocha OJ, et al. Detection of dengue virus neutralizing antibodies in bats from Costa Rica and Ecuador. J Med Entomol. 2000; 37: 965-967.
[12] Calderon A, Guzman C, Mattar S, et al. Dengue Virus in Bats from Cordoba and Sucre, Colombia. Vector Borne Zoonotic Dis. 2019; 19: 747-51.
[13] Weaver SC. Prediction and prevention of urban arbovirus epidemics: A challenge for the global virology community. Antiviral Res. 2018; 156: 80-84.
[14] Guzman MG, Harris E. Dengue. Lancet. 2015; 385: 453-465.
[15] Shu PY, Huang JH. Current advances in dengue diagnosis. Clin Diagn Lab Immunol. 2004; 11: 642-650.
[16] Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339: b2700.
[17] McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016; 75: 40-46.
[18] Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan - a web and mobile app for systematic reviews. Systematic Reviews 2016; 5: 1-10.
[19] Alarcón-Braga EA, Hernandez-Bustamante EA, Salazar-Valdivia FE, et al. Acceptance towards COVID-19 vaccination in Latin America and the Caribbean: A systematic review and meta-analysis. Travel Med Infect Dis. 2022; 49: 102369.
[20] Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021; 21.
[21] Hunter J, Saratzis A, Sutton A, Boucher R, Sayers R, Bown M. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014; 67: 897-903.
[22] de Thoisy B, Dussart P, Kazanji M. Wild terrestrial rainforest mammals as potential reservoirs for flaviviruses (yellow fever, dengue 2 and St Louis encephalitis viruses) in French Guiana. Trans R Soc Trop Med Hyg. 2004; 98: 409-412.
[23] Zabala R, Devesa M, Loureiro CL, Pernalete JM, Henríquez A, Liprandi F, et al. Prevalence of antibodies to dengue virus, hepadnavirus and rotavirus in non-human primates. Acta Científica Venezolana. 2006; 57: 22-27.
[24] Aguilar-Setien A, Romero-Almaraz ML, Sanchez-Hernandez C, et al. Dengue virus in Mexican bats. Epidemiol Infect. 2008; 136: 1678-83.
[25] Eastwood G, Sang RC, Guerbois M, Taracha ELN, Weaver SC. Enzootic Circulation of Chikungunya Virus in East Africa: Serological Evidence in Non-human Kenyan Primates. Am J Trop Med Hyg . 2017; 97: 1399-1404.
[26] Kading RC, Borland EM, Cranfield M, Powers AM. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo basin. J Wildl Dis. 2013; 49: 587-99.
[27] Machain-Williams C, López-Uribe M, Talavera-Aguilar L, et al. Serologic evidence of flavivirus infection in bats in the Yucatan Peninsula of Mexico. J Wildl Dis. 2013; 49: 684-689.
[28] Kilbourn AM, Karesh WB, Wolfe ND, Bosi EJ, Cook RA, Andau M. Health evaluation of free-ranging and semi-captive orangutans (Pongo pygmaeus pygmaeus) in Sabah, Malaysia. J Wildl Dis. 2003; 39: 73-83.
[29] Vicente-Santos A, Moreira-Soto A, Soto-Garita C, et al. Neotropical bats that co-habit with humans function as dead-end hosts for dengue virus. PLoS Negl Trop Dis. 2017; 11: e0005537.
[30] Kading RC, Kityo RM, Mossel EC, et al. Neutralizing antibodies against flaviviruses, Babanki virus, and Rift Valley fever virus in Ugandan bats. Infect Ecol Epidemiol. 2018; 8: 1439215.
[31] de Silva AM, Dittus WP, Amerasinghe PH, Amerasinghe FP. Serologic evidence for an epizootic dengue virus infecting toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. Am J Trop Med Hyg. 1999; 60: 300-306.
[32] Cigarroa-Toledo N, Talavera-Aguilar LG, Baak-Baak CM, et al. Serologic evidence of Flavivirus infections in peridomestic rodents in Merida, Mexico. J Wildl Dis. 2016; 52: 168-172.
[33] de Oliveira-Filho EF, Oliveira RAS, Ferreira DRA, et al. Seroprevalence of selected flaviviruses in free-living and captive capuchin monkeys in the state of Pernambuco, Brazil. Transbound Emerg Dis. 2018; 65: 1094-1097.
[34] Sotomayor-Bonilla J, Chaves A, Rico-Chávez O, Rostal MK, Ojeda-Flores R, Salas-Rojas M. Short report: Dengue virus in bats from southeastern Mexico. Am J Trop Med Hyg. 2014; 91: 129-131.
[35] Sotomayor-Bonilla J, García-Suárez O, Cigarroa-Toledo N, et al. Survey of mosquito-borne flaviviruses in the Cuitzmala River Basin, Mexico: do they circulate in rodents and bats? Trop Med Health. 2018; 46: 35.
[36] Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001; 64: 310-316.
[37] Diallo M, Ba Y, Sall AA, et al. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999-2000: entomologic findings and epidemiologic considerations. Emerg Infect Dis. 2003; 9: 362-367.
[38] Fagbami AH, Monath TP, Fabiyi A. Dengue virus infections in Nigeria: a survey for antibodies in monkeys and humans. Trans R Soc Trop Med Hyg. 1977; 71: 60-65.
[39] Dolz G, Chaves A, Gutiérrez-Espeleta GA, Ortiz-Malavasi E, Bernal-Valle S, Herrero MV. Detection of antibodies against flavivirus over time in wild non-human primates from the lowlands of Costa Rica. Plos One 2019; 14: e0219271.
[40] Inoue S, Morita K, Matias RR, et al. Distribution of three arbovirus antibodies among monkeys (Macaca fascicularis) in the Philippines. J Med Primatol. 2003; 32: 89-94.
[41] Peiris JS, Dittus WP, Ratnayake CB. Seroepidemiology of dengue and other arboviruses in a natural population of toque macaques (Macaca sinica) at Polonnaruwa, Sri Lanka. J Med Primatol 1993;22:240-5.
[42] Rudnick A. Studies of the ecology of Dengue in Malaysia: a preliminary report. J Med Entomol. 1965; 2: 203-208.
[43] Catenacci LS, Ferreira M, Martins LC, et al. Surveillance of Arboviruses in Primates and Sloths in the Atlantic Forest, Bahia, Brazil. Ecohealth. 2018; 15: 777-791.
[44] Moreira-Soto A, Carneiro IO, Fischer C, et al. Limited evidence for infection of urban and peri-urban nonhuman primates with Zika and Chikungunya viruses in Brazil. mSphere. 2018; 3.
[45] Kato F, Ishida Y, Kawagishi T, Kobayashi T, Hishiki T, Miura T, et al. Natural infection of cynomolgus monkeys with dengue virus occurs in epidemic cycles in the Philippines. J Gen Virol. 2013; 94: 2202-2207.
[46] Rosen L. Observations on the epidemiology of dengue in Panama. Am J Hyg. 1958; 68: 45-58.
[47] Hemme RR, Lopez-Ortiz R, Garcia BR, et al. Serological evidence of infection with endemic human pathogens among Free-Ranging Old World Monkeys in Puerto Rico. Am J Trop Med Hyg. 2016; 94: 1095-1099.
[48] Yuwono J, Suharyono W, Koiman I, Tsuchiya Y, Tagaya I. Seroepidemiological survey on dengue and Japanese encephalitis virus infections in Asian monkeys. Southeast Asian J Trop Med Public Health. 1984; 15: 194-200.
[49] Zhang H, Yang X, Li G. Detection of dengue virus genome RNA in some kinds of animals caught from dengue fever endemic areas in Hainan Island with reverse transcription-polymerase chain reaction. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1998; 12: 226-228.
[50] Abundes-Gallegos J, Salas-Rojas M, Galvez-Romero G, et al. Detection of Dengue virus in bat flies (Diptera: Streblidae) of common vampire bats, Desmodus rotundus, in Progreso, Hidalgo, Mexico. Vector Borne Zoonotic Dis. .2018; 18: 70-73.
[51] Bittar C, Machado RRG, Comelis MT, et al. Lack of serological and molecular evidence of arbovirus infections in bats from Brazil. Plos One. 2018; 13: e0207010.
[52] Cabrera-Romo S, Max Ramirez C, Recio-Tótoro B, et al. No evidence of Dengue virus infections in several species of bats captured in Central and Southern Mexico. Zoonoses Public Health. 2016; 63: 579-583.
[53] Irving AT, Rozario P, Kong PS, et al. Robust dengue virus infection in bat cells and limited innate immune responses coupled with positive serology from bats in IndoMalaya and Australasia. Cell Mol Life Sci. 2020; 77: 1607-1622.
[54] Kaul HN, Venkateshan CN, Mishra AC, Modi GB, Ghosh SN. Serological evidence of arbovirus activity in birds and small mammals in Japanese encephalitis affected areas of Bankura district, West Bengal. Indian J Med Res. 1976; 64: 1735-1739.
[55] Stone D, Lyons AC, Huang YS, et al. Serological evidence of widespread exposure of Grenada fruit bats to Chikungunya virus. Zoonoses Public Health. 2018; 65: 505-511.
[56] Ramos BA, Chiang JO, Martins LC, et al. Clinical and serological tests for arboviruses in free-living domestic pigeons (Columba livia). Mem Inst Oswaldo Cruz. 2017; 112: 532-536.
[57] Thongyuan S, Kittayapong P. First evidence of dengue infection in domestic dogs living in different ecological settings in Thailand. PLoS One. 2017; 12: e0180013.
[58] Price JL. Serological evidence of infection of Tacaribe virus and arboviruses in Trinidadian bats. Am J Trop Med Hyg. 1978; 27: 162-167.
[59] Kolman JM, Folk C, Hudec K, Reddy GN. Serologic examination of birds from the area of southern Moravia for the presence of antibodies against arboviruses of the groups Alfa, Flavo, Uukuniemi, Turlock and Bunyamwera supergroup. II. Wild living birds. Folia Parasitol (Praha). 1976; 23: 251-255.
[60] Ghosh SN, Rajagopalan PK, Singh GK, Bhat HR. Serological evidence of arbovirus activity in birds of KFD epizootic - epidemic area, Shimoga District, Karnataka, India. Indian J Med Res. 1975; 63: 1327-1334.
[61] Kalimuddin M, Narayan KG, Choudhary SP. Serological evidence of Japanese encephalitis virus activity in Bihar. Int J Zoonoses. 1982; 9: 39-44.
[62] Pauvolid-Corrêa A, Campos Z, Juliano R, Velez J, Nogueira RM, Komar N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS Negl Trop Dis. 2014; 8: e2706.
[63] Albanese M, Di Cuonzo G, Randazzo G, Srihongse S, Tringali G. Survey for Arbovirus antibodies in domestic animals of western Sicily. Ann Sclavo 1971; 13: 641-647.
[64] Okia NO, George PV, Tukei PM, et al. Arbovirus survey in wild birds in Uganda. East Afr Med J. 1971; 48: 725-731.
[65] Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983; 77: 442-445.
[66] Hussen MO, Sayed ASM, Abushahba MFN. Sero-epidemiological study on Dengue fever virus in humans and camels at Upper Egypt. Vet World 2020; 13: 2618-2624.
[67] Contigiani MS, Fernández C, Spinsanti LI, Díaz GE. Prevalence of Flavivirus antibodies in Alouatta caraya primate autochthonous of Argentina. Medicina (B Aires). 2000; 60: 348-50.
[68] Valentine MJ, Ciraola B, Aliota MT, Vandenplas M, Marchi S, Tenebray B, et al. No evidence for sylvatic cycles of Chikungunya, Dengue and Zika viruses in African green monkeys (Chlorocebus aethiops sabaeus) on St. Kitts, West Indies Parasit Vectors 2020; 13: 540.
[69] Loach TR, Narayan KG, Choudhary SP. Serological evidence of persistence of Japanese encephalitis virus activity in Bihar, India. Int J Zoonoses. 1983; 10: 7-14.
[70] Mall MP, Kumar A, Malik SV. Sero-positivity of domestic animals against Japanese encephalitis in Bareilly area, U.P. J Commun Dis. 1995; 27: 242-246.
[71] O?Connor JL, Rowan LC, Lawrence JJ. Relationships between the flying fox (genus Pteropus) and arthropod-borne fevers of North Queensland. Nature. 1955; 176: 472.
[72] Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004; 2: 789-801.
[73] Valentine MJ, Murdock CC, Kelly PJ. Sylvatic cycles of arboviruses in non-human primates. Parasites & Vectors. 2019; 12: 463.
[74] Calderón A, Guzmán C, Oviedo-Socarras T, Mattar S, Rodríguez V, Castañeda V, et al. Two Cases of Natural Infection of Dengue-2 Virus in Bats in the Colombian Caribbean. Trop Med Infect Dis. 2021; 6.
[75] Sotomayor-Bonilla J, Chaves A, Rico-Chávez O, et al. Dengue virus in bats from southeastern Mexico. Am J Trop Med Hyg. 2014; 91: 129-131.
[76] Bonilla-Aldana DK, Jimenez-Diaz SD, Arango-Duque JS, et al. Bats in ecosystems and their Wide spectrum of viral infectious potential threats: SARS-CoV-2 and other emerging viruses. Int J Infect Dis. 2021; 102: 87-96.
[77] Gwee SXW, St John AL, Gray GC, Pang J. Animals as potential reservoirs for dengue transmission: A systematic review. One Health. 2021; 12: 100216.
[78] Woodruff DS, Turner LM. The Indochinese-Sundaic zoogeographic transition: a description and analysis of terrestrial mammal species distributions. J Biogeography. 2009; 36: 803-821.
[79] Flies EJ, Lau CL, Carver S, Weinstein P. Another emerging mosquito-borne disease? Endemic ross river virus transmission in the absence of marsupial reservoirs. BioScience. 2018; 68: 288-293.
[80] Kek R, Hapuarachchi HC, Chung CY, et al. Feeding host range of Aedes albopictus (Diptera: Culicidae) demonstrates its opportunistic host-seeking behavior in rural Singapore. J Med Entomol. 2014; 51: 880-884.
[81] Barrera R, Bingham AM, Hassan HK, Amador M, Mackay AJ, Unnasch TR. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus (Diptera: Culicidae) in rural Puerto Rico. J Med Entomol. 2012; 49: 917-921.
[82] Cardona-Ospina JA, Arteaga-Livias K, Villamil-Gómez WE, Pérez-Díaz CE, Katterine Bonilla-Aldana D, Mondragon-Cardona Á, et al. Dengue and COVID-19, overlapping epidemics? An analysis from Colombia. J Med Virol. 2021; 93: 522-527.