Le Infezioni in Medicina, n. 2, 138-147, 2024
doi: 10.53854/liim-3202-3
REVIEWS
Anti-infective management of infected skin ulcers
Stefano Di Bella1, Roberto Luzzati1, Filippo Mearelli2, Giovanni Papa1, Luca Spazzapan3, Alessio Nunnari2, Francesco D’Aleo4, Carmelo Papola4, Luigi Principe4
1Clinical Department of Medical, Surgical, and Health Sciences, Trieste University, Trieste, Italy;
2Internal Medicine Unit, Trieste University Hospital (ASUGI), Trieste, Italy;
3Plastic Surgery Unit, Trieste University Hospital (ASUGI), Trieste, Italy;
4Clinical Microbiology and Virology Unit, Great Metropolitan Hospital “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy
Article received 21 February 2024, accepted 19 April 2024
Corresponding author
Stefano Di Bella
E-mail: stefano932@gmail.com
SummaRY
Infected skin ulcers represent a frequent and intricate clinical challenge, necessitating prompt and comprehensive multidisciplinary interventions to avert complications. Anti-infective therapy constitutes a cornerstone in the therapeutic paradigm. This manuscript delineates our approach to anti-infective management of infected ulcers, encompassing insights into clinical classifications, diagnostic features, exampless of early clinical decision-making in anti-infective treatment, comprehensive evaluation of infectious diseases encompassing host clinical considerations and potential interventions, determination of antibiotic therapy duration, methodologies for assessing clinical response, identification of potential causes for lack of clinical response, as well as strategies for outpatient parenteral antibiotic therapy and a diagnostic and therapeutic algorithm.
Keywords: Ulcers, infection, antibiotic therapy, microbiology.
INTRODUCTION
Infected skin ulcers are a common clinical problem with a significant impact on public health. Ulcers more commonly involve lower-extremities that are anatomically more prone to complications of vascular disorders, neuropathies and traumas. It is estimated that lower-extremity ulcers have a prevalence of 1-2% of the population in western countries [1, 2]. When facing ulcers, it is of fundamental importance to recognize if they are infected, especially in diabetic patients. Indeed, diabetic foot ulcers are more prone to infection, and more than half of them are recognized as clinically infected at the time of the patient’s presentation to the clinician [3, 4]. Amputation is experienced in 20% of diabetic foot ulcers and is linked to a high risk of death in the subsequent years [5]. A prompt identification of infection and an adequate, patient-tailored, anti-infective therapy can make the difference, by avoiding the amputation. We provide an opinion paper based on a synthesis of both historical and contemporary literature evidence. Papers deemed worthy by the panel of authors were referenced to discuss the various paragraphs. Below we discuss the main issues of infected ulcers from an infectious diseases point of view.
Classifications
Several classifications for skin ulcers exist and almost always apply to diabetic foot ulcers.
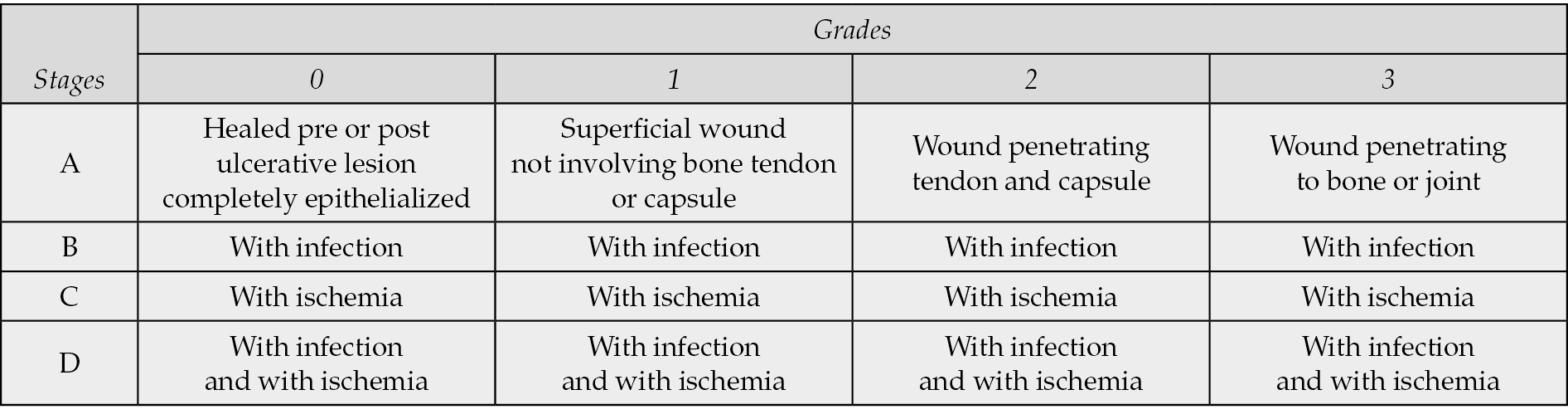
An “orthodox classification” has been provided by the Infectious Diseases Society of America (IDSA) and the International Working Group on the Diabetic Foot (IWGDF) (Table 1). This classification adopts 4 categories of lesions: uninfected, mild infection, moderate infection and severe infection [6].
Another widely used classification, simple and well-performing in prognostic terms, is the Texas classification (Table 2) [7]. This classification consists of a combined matrix of 4 grades (related to the depth of the wound) and 4 stages (related to the presence or absence of infection or ischemia). The classification successfully predicts a correlation of the likelihood of complications in patients with higher stages and grades and a significantly higher amputation rate in wounds deeper than superficial ulcers [8].
Table 1 - IDSA/IWGDF classification of skin ulcers.
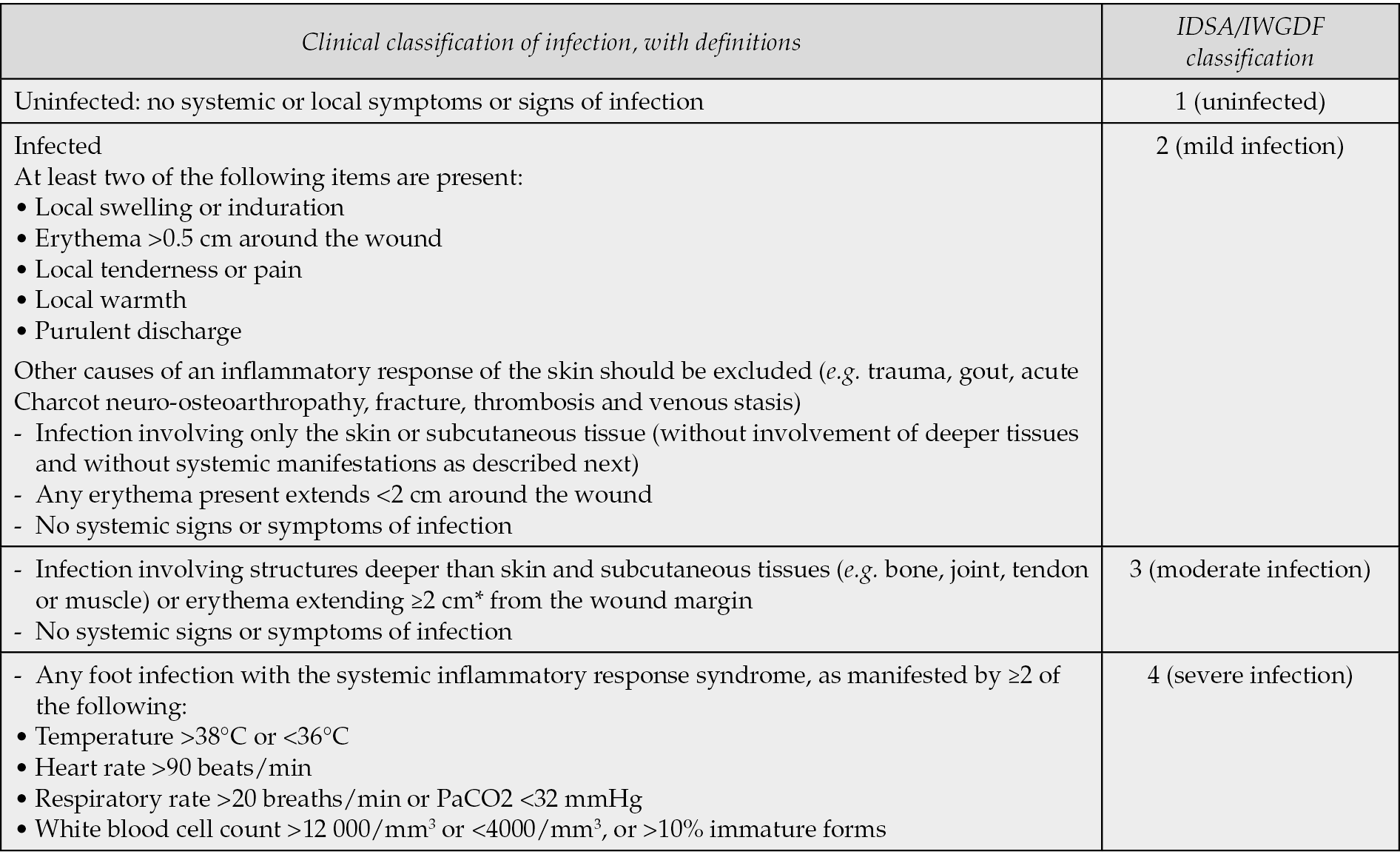
Table 2 - University of Texas classification system for skin ulcers.
Microbiology
The majority of acute infections of lower-extremity ulcers are caused by Gram-positive bacteria, especially staphylococci [9]. On the other hand, chronic infections of ulcers are often polymicrobic with Gram-positive, Gram-negative and anaerobic bacteria [10]. It is recommended to collect tissue samples for microbiology analysis (culture) before antibiotic therapy, especially in patients without systemic involvement. It is equally important to stress that for clinically uninfected ulcers, collecting a specimen for culture is not recommended [11-13]. Staphylococcus aureus and Pseudomonas aeruginosa are the most commonly isolated etiological agents from chronic lower limb skin ulcers. S. aureus is often detected in the upper layers of the ulcers, while P. aeruginosa is found in the deeper layers [14]. In chronic ulcers, the elastase produced by P. aeruginosa degrades immunoglobulin G and complement system components, contributing to ulcer chronicity. It is also important to consider the role played by biofilms in chronic non-healing ulcers, which impair the effectiveness of antibiotic action, promote localized tissue hypoxia, reduce the availability of oxygen required for the normal healing process, and lead to significantly increased pathogen minimum inhibitory concentrations (MICs) that may contribute to therapeutic failure [15].
Particular infectious causes of ulcers
Skin ulcers can sometimes underlie diseases different from vasculopathy, neuropathy and trauma. Vasculitis is one of the major diseases to be considered in differential diagnosis. From a strictly infectious diseases point of view, there are relatively uncommon diseases (that will be here only mentioned) that need to be taken into account when facing ulcerated skin lesions: cutaneous anthrax, chromo(blasto)mycosis, diphtheria, Francisella tularensis, leishmaniasis, mycetoma, mycobacterial diseases (e.g. Mycobacterium leprae, M. marinum, M. tuberculosis, M. ulcerans), sporotrichosis and tertiary syphilis (gumma) [16, 17].
Diagnostics
Macroscopic observation and clinical examination of the ulcer, the surrounding area and the patient in its entirety are the first fundamental steps to judge an ulcer infected or uninfected and to assess the possibility of systemic progression. The main local signs of infection to consider are: local warmth, erythema, tenderness or pain, swelling and purulent discharge [10]. The secondary signs are: nonpurulent secretions, friable or discolored granulation tissues, undermining of wound edges and foul odor [11].
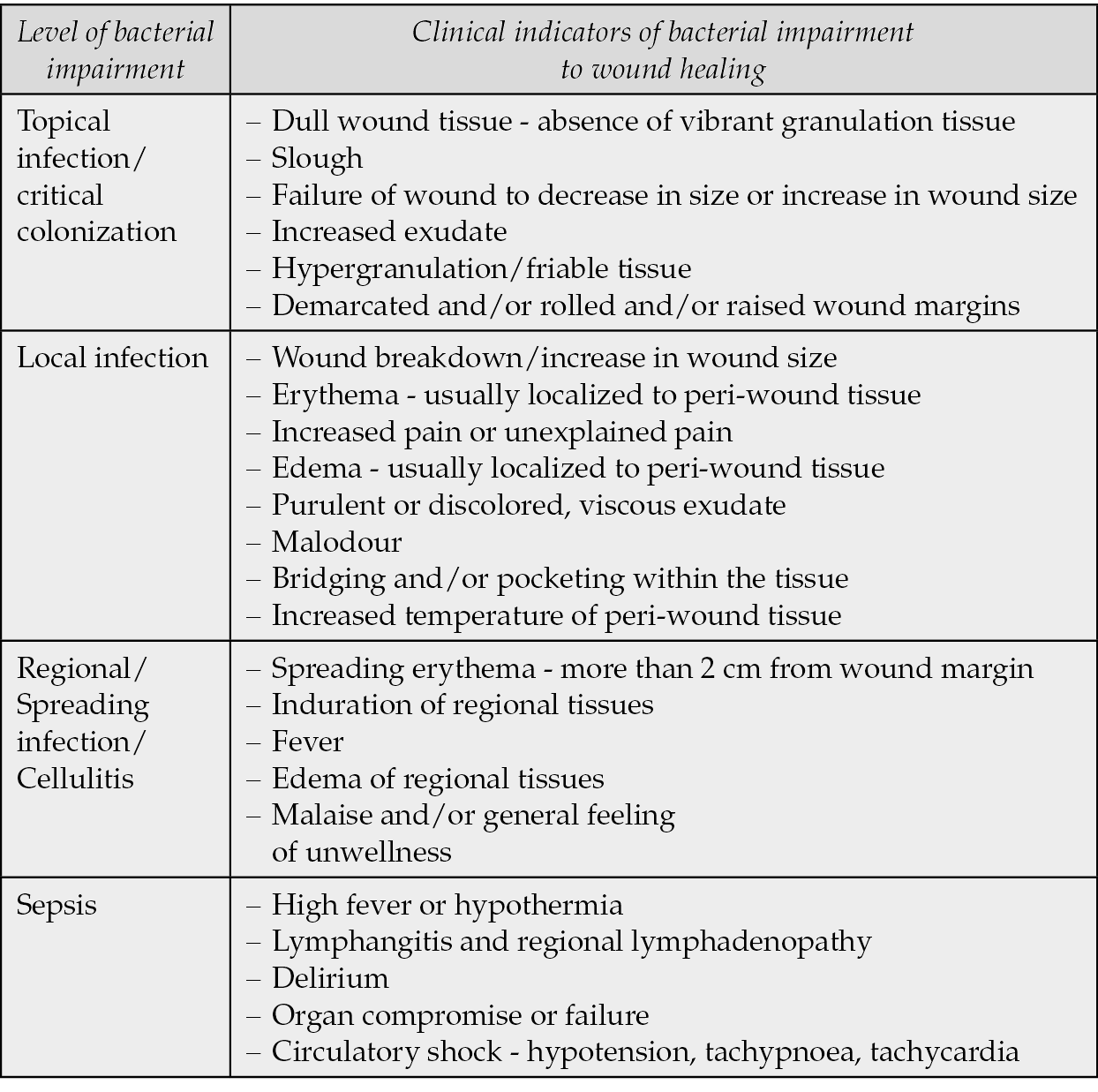
Many experts conceive the ulcer infection as a process that follows these steps: contamination -> colonization -> topical infection (critical colonization) -> local infection -> regional/spreading infection/cellulitis -> sepsis. With this in mind the Australian Wound Management Association (AWMA) provided clinical indicators of wound infection (Table 3), by specifying that “since bacterial impairment of wound healing is a continuum… worsening infection may or may not include some or all of the factors” [18].
Table 3 - Clinical indicators of infection according to AWMA.
Once the ulcer has been judged clinically infected, a prompt and accurate debridement of necrotic tissues and slough should be performed and tissue biopsies from the base of the ulcer should be collected. If diagnostic issues exist (e.g. vasculitic ulcers), it can be considered to send half of the bioptic material to the histopathology laboratory.
Regarding the type of samples, an old debate exists between swab and tissue biopsies, derived from some evidence showing that correctly performed swabs could reach sensitivity and specificity of tissue samples. We (and many experts) suggest preferring tissue biopsies in order to limit the possibility of detecting bacteria colonizing the superficial portion of the ulcer. In fact, the quality of the sample obtained influences whether the isolate can be considered a true pathogen rather than a colonizer and tissues biopsies after thorough debridement are considered the most valuable samples [19, 20]. Some microbiology laboratories can determine the quantitative count of organisms per gram of tissue, but this is rarely necessary for clinical situations [21].
Examples of early clinical reasoning/approach
- Disease extent and severity assessment
It is fundamental to figure out if the infection is only local or if systemic involvement signs exist, e.g.: fever (with or without chills), leukocytosis, expanding erythema and lymphangitis. A very high C-reactive protein should increase the suspicion of systemic involvement. Looking at the evolution possibility, it is important to exclude the progression toward a severe necrotizing infection, usually characterized by the presence of crepitus, bullae and extensive necrosis. Pain out of proportion to clinical signs should generate suspicion; LRINEC score could help in establishing pre-test probability, and an urgent computed tomography scan or magnetic resonance imaging should be performed to exclude a necrotizing infection (e.g. fasciitis) [22]. Necrotizing soft-tissue infections are life-threatening conditions and require an urgent consultation with a surgeon (e.g., vascular, general, orthopedic).
- Odour
Odour is another factor that can help clinicians, especially if a marked change is noticed from the patient and/or from their caregivers/health care professionals. A foul odour emergence is suggestive for infection (change in flora equilibrium with a pathogenic bacterial predominance over commensals/colonizers and high bacterial load) or for superinfection with anaerobes [23].
- Excluding bone involvement
We suggest to maintain a high suspicion index in order to assess if infection of ulcers (especially those close to bone prominences such as ankles and feet) has progressed in an underlying osteomyelitis (Figure 1). A concomitant osteomyelitis would significantly change the therapeutic approach:
- it would need a more aggressive debridement;
- it would benefit from a deep sampling (bone biopsy);
- it would require a longer antibiotic therapy (6 weeks if the bone is vital).
From an imaging point of view, when suspecting an underlying osteomyelitis, the first step is the plain X-ray but this is often followed by magnetic resonance imaging (for osteomyelitis in diabetic foot: sensitivity 90%, specificity 85%) [24, 25]. Histopathology examination of the bone can be useful to confirm the osteomyelitis, since the probe-to-bone not necessarily means that the bone is affected [26].
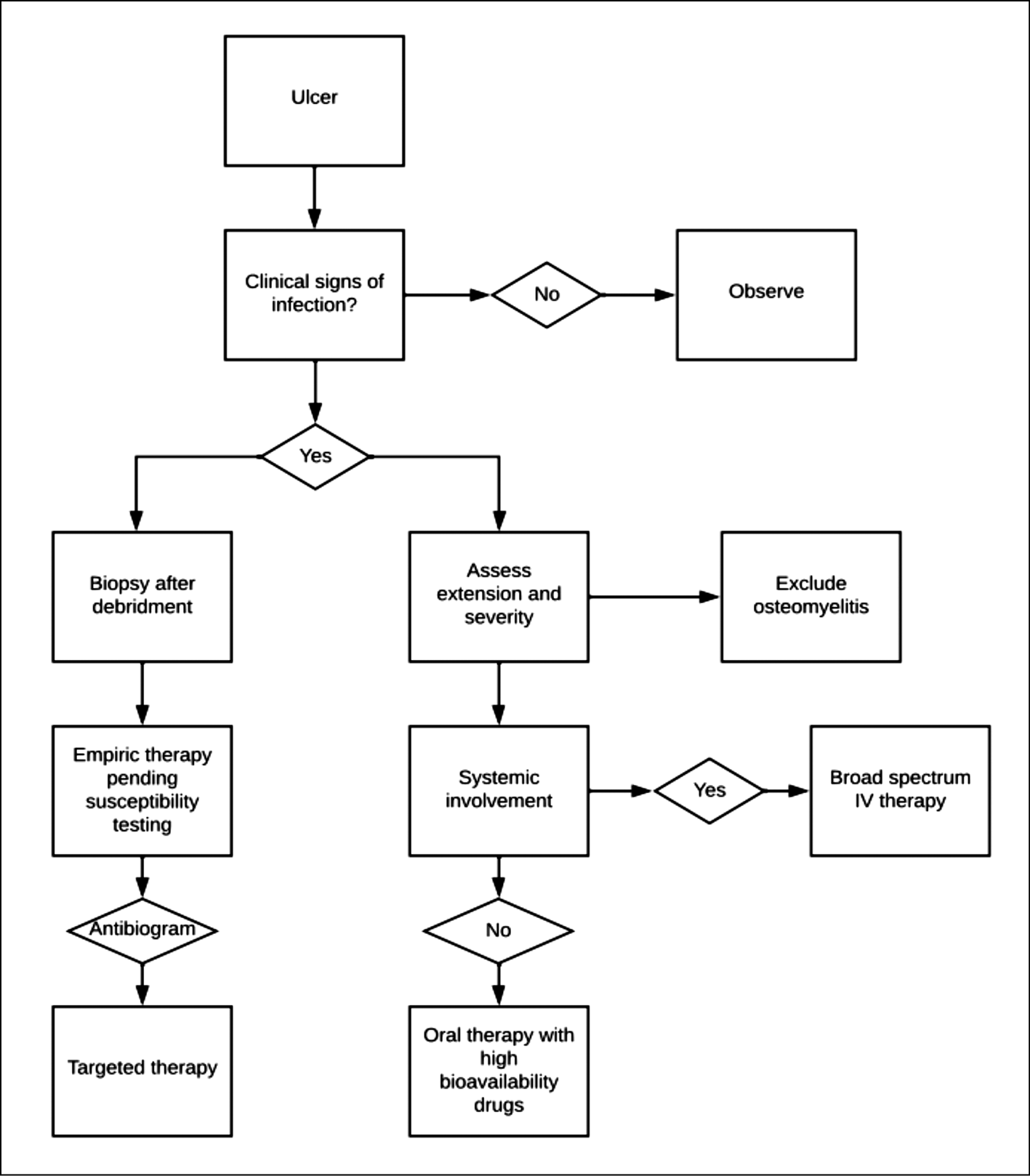
Figure 1 - Reasoned diagnostic and therapeutic algorithm.
- When to consider anaerobic coverage?
Anaerobes are difficult to cultivate in standard laboratories; therefore their absence of growth does not exclude their involvement. Their pathogenic role is still debated but some casistics have shown that the isolation of anaerobes was higher in those who subsequently underwent a lower extremity amputation [27]. The rate of anaerobic infection does appear to be highest in ischemic or necrotic ulcers, where the impaired blood supply and low redox potential may facilitate their proliferation [27]. An appearance of foul odor may suggest anaerobic superinfection.
Anti-infective treatment
- When to treat
Only clinically infected ulcers should be treated with anti-infective agents, in fact data from the literature have not demonstrated advantages from prophylactic therapies or treatment of noninfected ulcers [28]. Usually, although it is recommended to postpone antibacterial therapy until tissue sampling, it is not recommended to wait for antimicrobial susceptibility testing, especially in diabetic patients. Therefore, the optimum would be to start antibiotics after bioptic tissues have been taken, pending bacterial identification and antibiotic susceptibility testing (Figure 1). An exception can be made in previously treated patients at risk for multidrug-resistant bacteria.
- Topic antimicrobials: more shadows than lights
Regarding topical antibiotics, there are more shadows than lights. A commonly used compound, with adequate literature data is silver sulfadiazine, however the Canadian Agency for Drugs and Technologies in Health in 2017 stated “There is currently insufficient evidence to recommend the use of silver sulfadiazine for the treatment of infected or contaminated chronic wounds” [29]. Apart from a disappointing efficacy, there is also concern that topical antibiotics may further promote the emergence of bacterial resistance [30]. Some hope arises from bioengineered honey, which has shown activity against Gram-negative bacterial biofilms [31].
- How to cover anaerobes?
Although the pathogenic role of anaerobes in ulcers is still a matter of debate, it has been demonstrated that anaerobes represented 49% of the total microbial composition in infected leg ulcers compared with 36% in noninfected leg ulcers [32]. Baron et al. suggest to always consider anaerobes in feet ulcers [19]. Anaerobes more commonly isolated from infected ulcers are Bacteroides spp. and Peptostreptococcus spp. [27]. Antibiotic resistances among anaerobes are increasing (especially among Bacteroides spp.), but a betalactam with a betalactamase inhibitor (e.g., amoxicillin/clavulanate, piperacillin/tazobactam) remains a good option [33].
- Systemic or not?
It is important to understand this crucial point: in the “systemic” patient it is preferred to direct the therapy toward hydrophilic drugs to maximize the activity on potential bacteremia, while in localized infections it is preferred the use of lipophilic drugs to achieve a greater tissue penetration. For example, in systemic patients we suggest using daptomycin instead of linezolid to cover methicillin-resistant Staphylococcus aureus (MRSA). On the contrary, in local infections we suggest using doxycycline instead of amoxicillin/clavulanate against methicillin-susceptible S. aureus. Age ≥65 years, involvement of non-lower extremities, liver cirrhosis, and systemic inflammatory response syndrome are recognized risk factors for bacteremia development in adults with cellulitis [34, 35].
- Taking into account the minimum inhibitory concentration (MIC)
When antibiotic susceptibility testing becomes available, especially in monomicrobial infections, it is important to optimize the therapy taking into account the MIC of the different molecules by knowing the clinical breakpoint for a certain bacterium. This evaluation is helpful to increase the possibility of microbiological and clinical success, especially considering that very often the circulation is impaired, so it is much better to stay distant from the breakpoint.
- Oral absorption (mild/moderate cases)
In mild/moderate infection, if a molecule with high oral bioavailability is used in a patient with good gastrointestinal function, it is acceptable to start orally. This would usually led to less complications (phlebitis) and would allow care for the patient in an outpatient setting. In Table 4 is reported a list of antibiotics with good oral bioavailability.
Table 4 - Antibiotics with good oral bioavailability commonly used for infected ulcers [36, 37].
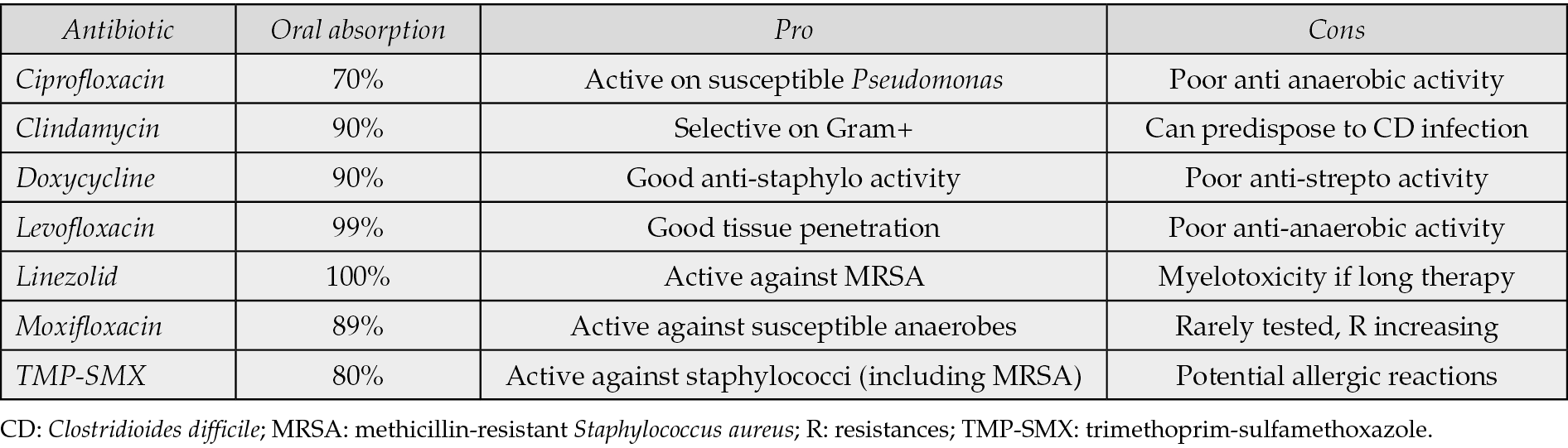
- Know your local epidemiology
An updated knowledge of the local epidemiology is fundamental; for example, in our hospital 90% of staphylococci are susceptible to doxycycline and trimethoprim-sulfamethoxazole, making them good options for anti staphylococcal empirical therapy in mild-moderate infections. This should be re-evaluated every year with susceptibility data collected by the microbiology laboratory. In general, if a microorganism has a resistance >20% for an antibiotic in a certain epidemiology, avoid this drug in empirical therapy.
- Local infections: rational antibiotic regimens
In mild to moderate infections in patients who have not recently received antibiotics, it is reasonable to start a therapy more focused to Gram-positive bacteria, often with MRSA coverage, since the majority of these patients have risk factors for MRSA [12]. We suggest the following regimens: doxycycline + clindamycin; doxycycline + cefazolin; linezolid. We use doxycycline (usually active against MRSA) in combination because it is poorly active against Streptococcus pyogenes. In patients without MRSA risk factors, amoxicillin/clavulanate is a reasonable empirical choice for not already “antibiotic experienced” patients.
- Systemic empiric antibiotic regimens
In patients with severe infections depending on the degree of systemic involvement intravenous therapy should be preferred and therapy should cover Gram-positive, Gram-negative (including Pseudomonas) and anaerobes, e.g., daptomycin + piperacillin/tazobactam (± fosfomycin) or daptomycin + meropenem.
- Antipseudomonal agents
Pseudomonas is a difficult-to-treat pathogen. Empirical coverage for Pseudomonas should be considered in patients who are colonized, particularly those with lower extremity ulcers or diabetic foot ulcers, as well as patients residing in regions with warm and humid climates [38]. The only orally active agent is ciprofloxacin but resistances are quite high. Commonly used antibiotics with antipseudomonal activity are: amikacin, cefepime, ceftolozane/tazobactam, meropenem and piperacillin/tazobactam. Cefepime, ceftolozane/tazobactam and piperacillin/tazobactam would allow continuous infusion [39]. Fosfomycin can be often used as partner drug, especially alongside cephalosporins [40, 41].
- Multidrug-resistant (MDR) bacteria
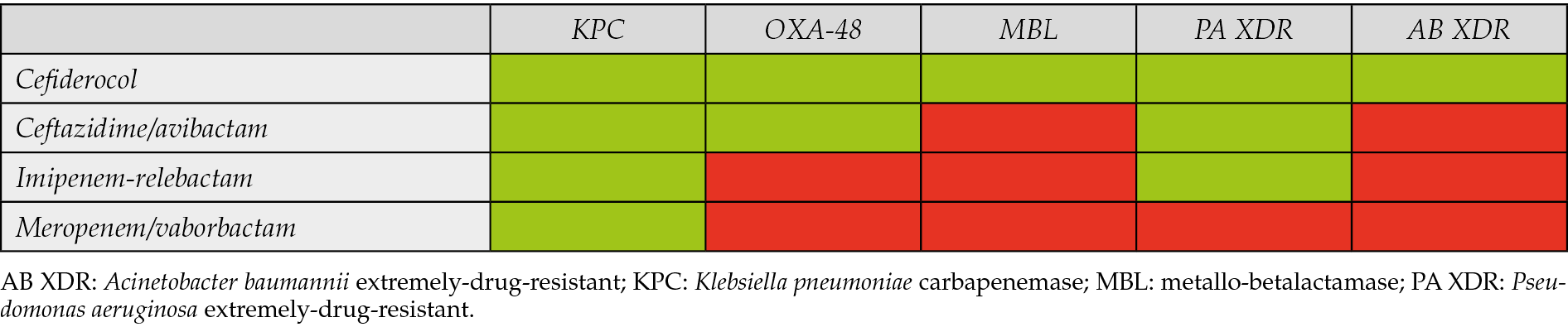
Multidrug resistant bacteria require case-by-case therapies. Combination regimens are often used and fosfomycin is often considered because of its synergistic potential. Linezolid is usually a good choice for Gram-positives while for Gram-negatives the last resources and their relative microbiological targets are shown in table 5, keeping in mind that MDR bacterial infections deserve a case-by-case evaluation, often driven by susceptibility testing.
Table 5 - New anti multi-drug resistant antibiotics and their microbiological targets.
- Long acting antibiotics
Recently, long-acting antibiotics have been approved for clinical use. Dalbavancin and oritavancin are sometimes considered for infected ulcers ± osteomyelitis in special situations (e.g., poor compliance). Both drugs have activity against Gram-positives (including MRSA), are approved for skin and soft tissue infections and have solid data also in patients with osteomyelitis [42, 43]. Therapeutic drug monitoring for both dalbavancin and oritavancin is being increasingly used in cases that need multidose administrations, although the interpretation of oritavancin serum levels is still an issue [44].
A reasoned diagnostic and therapeutic algorithm for the management of infected skin ulcers is provided in Figure 1.
Holistic infectious diseases evaluation: host clinical issues and potential countermeasures
When choosing an antimicrobial therapy, basic principles of pharmacokinetic/pharmacodynamic (PK/PD) should be kept in mind and adapted to the patient (liver and/or renal impairment, hypoalbuminemia, allergies, etc.). We describe several important factors that may influence the choice and that deserve to be evaluated below:
- advanced liver disease -> e.g. ascites -> altered volume of distribution, prefer cefotaxime to ceftriaxone, prefer meropenem to ertapenem,
- arterial vasculopathy -> difficulty of antibiotics to reach the target site -> prefer lipophilic drugs with good diffusion in soft tissues, e.g. quinolones, tetracyclines, linezolid,
- bone involvement -> prefer lipophilic drugs such as regimens including quinolones, consider rifampicin or fosfomycin as partner drugs,
- diabetes -> damaged microcirculation -> expect a slower response, prefer lipophilic drugs with good diffusion in soft tissues, e.g., quinolones, tetracyclines, linezolid),
- hypoalbuminemia -> especially negatively influence PK/PD of antibiotics with high protein-bound drugs, e.g. ertapenem, ceftriaxone (prefer meropenem and cefotaxime, respectively) [45],
- obesity -> PK/PD issues that can require dose adjustment, e.g. linezolid standard dosing may be inadequate, use actual body weight to calculate clindamycin dose,
- previous Clostridioides difficile infection -> prefer drugs with low colitis induction potential e.g. dalbavancin, doxycycline, linezolid, piperacillin/tazobactam,
- renal failure -> adjust posologies of hydrophilic drugs as needed, prefer lipophilic drugs e.g. minocycline.
Antibiotic therapy duration
For mild to moderate skin and soft tissue infections, 1-2 weeks of therapy is usually effective, for severe infections (systemic involvement) the length is usually protracted to 2 weeks (in selected cases of slow response in patients with peripheral artery disease 4 weeks). Antibiotics should not be continued through complete healing of the ulcer. If there is concomitant osteomyelitis therapy usually lasts 6 weeks (if the bone is judged viable) [46].
How to assess clinical response?
The majority of diabetic foot ulcers take a minimum of 20 weeks to heal [47, 48]. The ulcer area needs to be measured regularly. Apart from ulcer reduction, other signs that suggest amelioration are: pain decrease, malodour reduction, decrease of surrounding erythema and decrease of local temperature.
Any increase ≥20% between two measurements should be considered as an increase in wound size. If over a 4-week period of appropriate treatment (not only anti-infective), the ulcer size does not decrease by at least 20%, delayed healing should be noted [49].
Blood tests are also useful to confirm a trend, e.g. white cell count, C-reactive protein, erythrocyte sedimentation rate.
Possible causes of lack of clinical response
A non-exhaustive list of potential causes of clinical failure is herein reported:
- Antibiotic therapy directed toward a colonizer (real pathogen not identified).
- Underlying vascular failure too strong (limb ischemia).
- Necrotic tissue (unidentified) not removed.
- Bacterial resistances not detected or new resistances developed.
- Multiple bacterial phenotypes (e.g. multiple Pseudomonas populations).
- Reinfections with new bacteria.
- Poor therapy adherence (especially in outpatients).
Outpatient parenteral antibiotic therapy (OPAT)
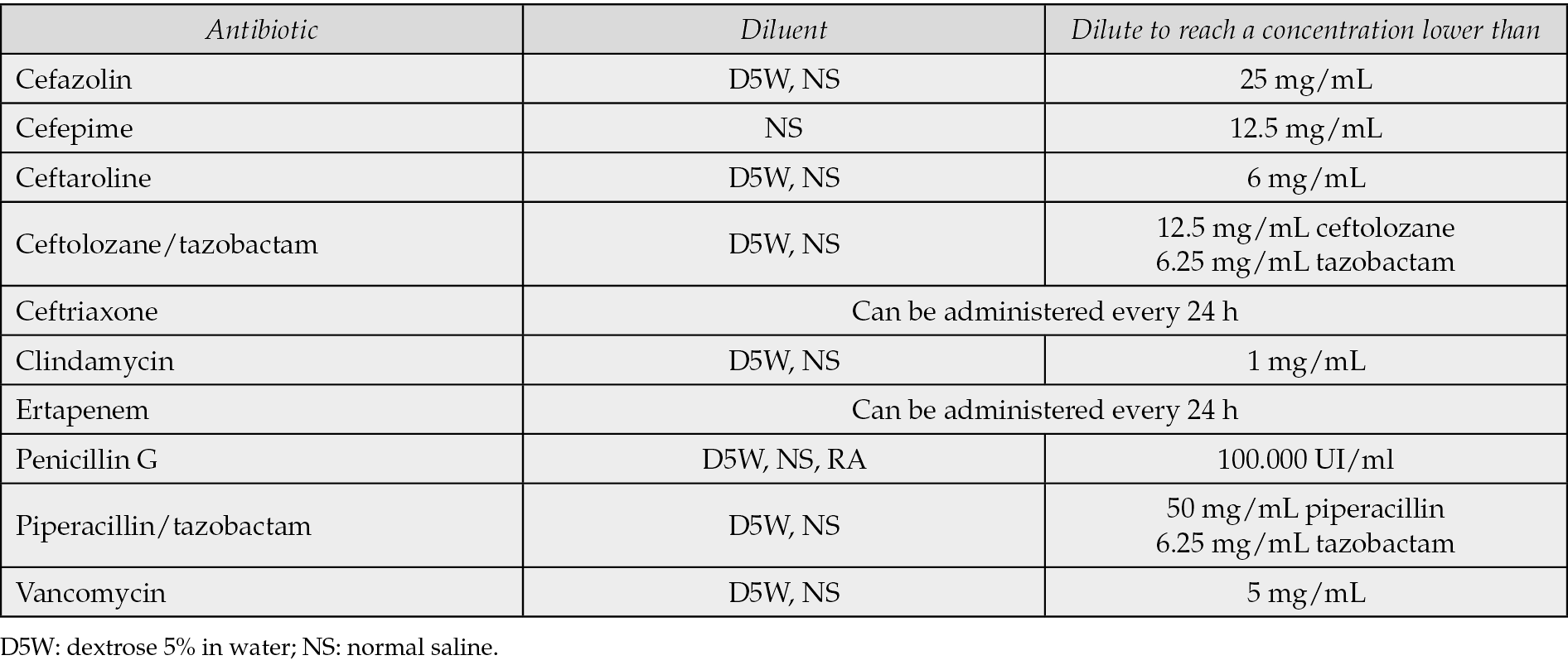
For patients who are not severely sick, but would benefit from intravenous therapy (e.g. impaired gastrointestinal absorption, few options for resistances) some antibiotics are suitable for outpatient parenteral antibiotic therapy (OPAT) (Table 6). Some of them can be administered once daily and the others can be administered in 24-h continuous infusion with elastomeric pumps (in this case the patients would usually need a medium-long term venous access such as Midline or PICC) [50].
Table 6 - Antibiotics suitable for OPAT [39].
CONCLUSION
Our work, while acknowledging its limitation as an opinion paper according to authors’ perspective, highlights the fact that the management of infected cutaneous ulcers presents a nuanced challenge, necessitating a multidisciplinary approach for optimal outcomes. A comprehensive clinical assessment forms the cornerstone, entailing a meticulous examination of key indicators to discern the infectious nature of an ulcer. Subsequent steps involve deep tissue sampling for microbial culture, facilitating a tailored therapeutic strategy that not only addresses isolated pathogens but also considers pertinent variables such as potential contaminations, the presence of fastidious microorganisms, and the formation of biofilms. Equally paramount is the diligent exclusion of underlying osseous involvement in equivocal cases, thus enabling the determination of the optimal duration for antibiotic therapy.
REFERENCES
[1] Alavi A, Sibbald RG, Phillips TJ, et al. What’s new: Management of venous leg ulcers: Treating venous leg ulcers. J Am Acad Dermatol. 2016; 74(4): 643-664.
[2] Margolis DJ, Bilker W, Knauss J, Baumgarten M, Strom BL. The incidence and prevalence of pressure ulcers among elderly patients in general medical practice. Ann Epidemiol. 2002; 12(5): 321-325.
[3] Lavery LA, Armstrong DG, Wunderlich RP, Mohler MJ, Wendel CS, Lipsky BA. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006; 29(6): 1288-1293.
[4] Esposito S, Noviello S, De Caro F, Boccia G. New insights into classification, epidemiology and microbiology of SSTIs, including diabetic foot infections. Infez Med. 2018; 26(1): 3-14.
[5] Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag. 2007; 3(1): 65-76.
[6] Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016; 32 (Suppl. 1): 45-74.
[7] Oyibo SO, Jude EB, Tarawneh I, Nguyen HC, Harkless LB, Boulton AJ. A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care. 2001; 24(1): 84-88.
[8] Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998; 21(5): 855-859.
[9] Dang CN, Prasad YDM, Boulton AJM, Jude EB. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003; 20(2): 159-161.
[10] Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and Management of Lower-Extremity Ulcers. N Engl J Med. 2018; 378(3): 302-303.
[11] Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012; 54(12): e132-173.
[12] Esposito S, Pagliano P, De Simone G, et al. Epidemiology, aetiology and treatment of skin and soft tissue infections: final report of a prospective multicentre national registry. J Chemother. 2022; 34(8): 524-533.
[13] Esposito S, Ascione T, Pagliano P. Management of bacterial skin and skin structure infections with polymicrobial etiology. Expert Rev Anti Infect Ther. 2019; 17(1): 17-25.
[14] Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015; 13(5): 605-613.
[15] Di Domenico EG, Oliva A, Guembe M. The Current knowledge on the pathogenesis of tissue and medical device-related biofilm infections. Microorganisms. 2022; 10(7): 1259.
[16] Zeegelaar JE, Faber WR. Imported tropical infectious ulcers in travelers. Am J Clin Dermatol. 2008; 9(4): 219-232.
[17] Markowicz M, Schötta AM, Penatzer F, Matscheko C, Stanek G, Stockinger H, et al. Isolation of Francisella tularensis from Skin Ulcer after a Tick Bite, Austria, 2020. Microorganisms. 2021; 9(7): 1407.
[18] Bacterial Impact on Wound Healing: From Contamination to. Infection. Aivailable at: https://awma.com.au/files/publications/2011_bacterial_impact_position_1.5.pdf. Accessed 2 August 2022.
[19] Baron EJ, Miller JM, Weinstein MP, Richter SS, Gilligan PH, Thomson RB Jr, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013; 57(4): e22-121.
[20] Esposito S, De Simone G, Gioia R, et al. Deep tissue biopsy vs. superficial swab culture, including microbial loading determination, in the microbiological assessment of Skin and Soft Tissue Infections (SSTIs). J Chemother. 2017; 29(3): 154-158.
[21] Nelson EA, O’Meara S, Craig D, et al. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess. 2006; 10(12): 1-221.
[22] Wong CH, Khin LW. Clinical relevance of the LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score for assessment of early necrotizing fasciitis. Crit Care Med. 2005; 33(7): 1677.
[23] Bowler PG, Davies BJ, Jones SA. Microbial involvement in chronic wound malodour. Journal of Wound Care. 1999; 8: 2016-2018.
[24] Kapoor A, Page S, Lavalley M, Gale DR, Felson DT. Magnetic resonance imaging for diagnosing foot osteomyelitis: a meta-analysis. Arch Intern Med. 2007; 167(2): 125-132.
[25] Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008; 47(4): 519-527.
[26] Wong D, Holtom P, Spellberg B. Osteomyelitis Complicating Sacral Pressure Ulcers: Whether or Not to Treat With Antibiotic Therapy. Clin Infect Dis. 2019; 68(2): 338-342.
[27] Charles PGP, Uçkay I, Kressmann B, Emonet S, Lipsky BA. The role of anaerobes in diabetic foot infections. Anaerobe. 2015; 34: 8-13.
[28] Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother. 2015; 16: 821-832.
[29] Cowling T, Jones S. Topical Antibiotics for Infected Wounds: A Review of the Clinical Effectiveness and Guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018.
[30] Cutting K, White R, Edmonds M. The safety and efficacy of dressings with silver - addressing clinical concerns. Int Wound J. 2007; 4(2): 177-184.
[31] Newby RS, Dryden M, Allan RN, Salib RJ. Antimicrobial activity of a novel bioengineered honey against non-typeable Haemophilus influenzae biofilms: an in vitro study. J Clin Pathol. 2018; 71(6): 554-558.
[32] Bowler PG, Davies BJ. The microbiology of infected and noninfected leg ulcers. Int J Dermatol. 1999; 38(8): 573-578.
[33] Di Bella S, Antonello RM, Sanson G, et al. Anaerobic bloodstream infections in Italy (ITANAEROBY): A 5-year retrospective nationwide survey. Anaerobe. 2022; 75: 102583.
[34] Lee CY, Kunin CM, Chang C, Lee SS, Chen YS, Tsai HC. Development of a prediction model for bacteremia in hospitalized adults with cellulitis to aid in the efficient use of blood cultures: a retrospective cohort study. BMC Infect Dis. 2016; 16(1): 581.
[35] Falcone M, Meier JJ, Marini MG, et al. Diabetes and acute bacterial skin and skin structure infections. Diabetes Res Clin Pract. 2021; 174: 108732.
[36] Cunha BA. Oral antibiotic therapy of serious systemic infections. Med Clin North Am. 2006; 90: 1197-1222.
[37] Principe L, Sanson G, Luzzati R, Aschbacher R, Pagani E, Luzzaro F, et al. Time to reconsider moxifloxacin anti-anaerobic activity? J Chemother. 2023; 35(4): 367-368.
[38] Veve MP, Mercuro NJ, Sangiovanni RJ, Santarossa M, Patel N. Prevalence and Predictors of Pseudomonas aeruginosa Among Hospitalized Patients With Diabetic Foot Infections. Open Forum Infect Dis. 2022; 9(7): ofac297.
[39] Di Bella S, Beović B, Fabbiani M, Valentini M, Luzzati R. Antimicrobial stewardship: from bedside to theory. Thirteen examples of old and more recent strategies from everyday clinical practice. Antibiotics (Basel). 2020; 9(7): 398.
[40] Antonello RM, Principe L, Maraolo AE, Viaggi V, Pol R, Fabbiani M, et al. Fosfomycin as partner drug for systemic infection management. A systematic review of its synergistic properties from in vitro and in vivo studies. Antibiotics (Basel). 2020; 9(8): 500.
[41] Pipitone G, Di Bella S, Maraolo AE, et al. Intravenous Fosfomycin for systemic Multidrug-Resistant Pseudomonas aeruginosa infections. Antibiotics (Basel). 2023; 12(12): 1653.
[42] Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2019; 6(1): 331.
[43] Van Hise NW, Petrak RM, Shah K, Diaz M, Chundi V, Redell M. Oritavancin versus daptomycin for osteomyelitis treatment after surgical debridement. Infect Dis Ther. 2024; 13(3): 535-547.
[44] Cafaro A, Barco S, Pigliasco F, et al. Therapeutic drug monitoring of glycopeptide antimicrobials: An overview of liquid chromatography-tandem mass spectrometry methods. J Mass Spectrom Adv Clin Lab. 2023; 31: 33-39.
[45] Zusman O, Farbman L, Tredler Z, et al. Association between hypoalbuminemia and mortality among subjects treated with ertapenem versus other carbapenems: prospective cohort study. Clin Microbiol Infect. 2015; 21(1): 54-58.
[46] Lipsky BA, Senneville É, Abbas ZG, et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020; 36 (Suppl. 1): e3280.
[47] Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008; 16(1): 19-22.
[48] Ince P, Game FL, Jeffcoate WJ. Rate of healing of neuropathic ulcers of the foot in diabetes and its relationship to ulcer duration and ulcer area. Diabetes Care. 2007; 30(3): 660-663.
[49] Bui UT, Finlayson K, Edwards H. The diagnosis of infection in chronic leg ulcers: A narrative review on clinical practice. Int Wound J. 2019; 16(3): 601-620.
[50] Fernández-Rubio B, Del Valle-Moreno P, Herrera-Hidalgo L, et al. Stability of antimicrobials in elastomeric pumps: a systematic review. Antibiotics (Basel). 2021; 11(1): 45.