Le Infezioni in Medicina, n. 2, 241-247, 2024
doi: 10.53854/liim-3202-12
CASE REPORTS
Prolonged watery diarrhea and malnutrition caused by Aliarcobacter butzleri (formerly Arcobacter butzleri): the first pediatric case in Croatia and a literature review
Anita Novak1, 2, Marija Vuko-Tokic´1, Vanda Žitko3, Marija Tonkic´1,2
1University of Split, School of Medicine Split, Croatia;
²Department of Microbiology, University Hospital of Split, Croatia;
3Department of Pediatrics, University Hospital of Split, Croatia
Article received 26 March 2024, accepted 13 May 2024
Corresponding author
Anita Novak
E-mail: anitanovak1@net.hr
SummaRY
Aliarcobacter butzleri (formerly Arcobacter butzleri), is a newly recognized Campylobacter-like emerging foodborne pathogen worldwide, usually causing gastrointestinal symptoms in young children.
A 4-year-old boy was admitted to the Department of Pediatrics, University Hospital of Split, Croatia, because of malnutrition, lost appetite and prolonged watery diarrhea. A comprehensive diagnostics, including biochemistry, haematology, allergology, microbiology and radiology, were performed. The only positive microbiology result was unexpected isolation of Aliarcobacter butzleri on selective media for Campylobacter, after 48 hours of incubation on 42°C, among microaerophilic atmosphere. Clinical course was favorable and after symptomatic therapy child was discharged in good clinical condition and normal peristalsis to home care, with the recommendation of taking high-protein preparations to improve nutritional status. In addition, we performed a literature review of clinical cases caused by Aliarcobacter butzleri infection.
The first report of Aliarcobacter butzleri isolated from stool sample in a 4-year old boy in Croatia, along with other clinical reports in literature, highlights the importance of standardisation and improvement of microbiological analysis, especially implementation of new methods for the identification of emerging pathogens.
Keywords: Aliarcobacter butzleri, Campylobacter-like bacteria, prolonged watery diarrhea, emerging foodborne pathogen, malnutrition.
INTRODUCTION
Aliarcobacter butzleri, formerly known as Arcobacter butzleri, is a newly reclassified bacterium, taxonomically closely related to genus Campylobacter. By 2020, 33 species have been included in the Aliarcobacter gen. nov., of which A. butzleri is described as potentially the most virulent species. The typical morphology seen in Gram staining smear is a Gram-negative, curved bacillus, 0.2-0.5 µm in diameter and 1-3 µm long [1-3].
It can be found in the environment as well as in main food sources, like plants (predominantly vegetables), seafood and food producing animal. A. butzleri is recognized as an emerging foodborne and waterborne human enteric pathogen worldwide and one of the 4 most frequently diagnosed Campylobacter-like bacteria associated with human infections [1, 4-6]. In comparison with bloody diarrhea caused by Campylobacter species, A. butzleri mostly causes prolonged watery diarrhea [2, 7]. Occasionally, A. butzleri may cause severe enteritis and septicaemia and the disease progression may be dose-dependent. Isolation of the A. butzleri from so many different sources suggests that transmission should be viewed in the light of the One Health concept [1, 6].
The lack of a standardized protocol for isolation and identification of A. butzleri complicates the monitoring of the incidence and the route of transmission. There are significant differences in microbiological methodology, e.g. usage of different nutrient media and incubation conditions for the cultivation of A. butzleri. Merga et al. compared five different cultivation techniques and concluded that the highest percentage of successful isolation of A. butzleri from the positive samples is only 70.7%. Arcobacter broth-mCCDA-Columbia Agar, a selective medium with antibiotics, proved to be the most sensitive and specific medium in this study [8].
Moreover, it seems that Aliarcobacter may grow upon wide range of temperature (from 15 to 42°C) and under different atmospheric conditions (aerobic, microaerophilic and anaerobic). This inconsistency in cultivation methodology complicates the interpretation of the results obtained from different researches [1, 8]. Consequently, we may assume that the prevalence of aliarcobacteriosis is bigger than we can prove at this moment. In recent research by Webb et al., the prevalence of A. butzleri in human stool samples is 60%, which is much higher in comparison to previous reports (25%) [9].
Aliarcobacteriosis, just like campylobacteriosis, is usually self-limited disease, treated symptomatically and no antibiotic treatment is needed. Antibiotics are recommended only in the case of severe infection or worsening of clinical symptoms. A. butzleri is usually susceptible to fluoroquinolones, macrolides, tetracyclines and aminoglycosides, while resistant to penicillins and cephalosporins [1, 4, 5, 10]. However, the growing trend of antimicrobial resistance in foodborne and waterborne pathogens is present worldwide and the antimicrobial resistance rates in Aliarcobacter changed over the time. The high resistance rates to fluoroquinolones, macrolides and aminoglycosides (up to 14.0, 39.8 and 12.9%) have been already documented in same studies [1, 10]. Moreover, multidrug-resistant strains (resistant to azithromycin, nalidixic acid and clindamycin) have been reported, which may become a new global threat in the future [11].
In this report, we aimed to describe the first case of prolonged watery diarrhea, complicated with malnutrition, caused by Aliarcobacter butzleri in a University Hospital of Split, Croatia. Furthermore, we performed a literature review to improve knowledge on clinical presentation, treatment and outcome of human aliarcobacteriosis.
CASE REPORT
A four-year-old boy with a history of abdominal pain and prolonged watery diarrhea was admitted to the Departmant of Pediatrics, University Hospital of Split, Croatia for the observation and treatment of prolonged diarrhea and consecutive malnutrition.
History of present illness
His symptoms started four weeks prior to admission with four to five watery stools per day, occasionally tinted with mucus, but without macroscopically visible blood. Hyperperistalsis followed almost every meal, so the child soon lost the appetite.
A month before current disease the boy was suffering from an acute febrile gastroenterocolitis but no microbiological diagnosis was made at that time. For this reason, development of post-infectious motility disorder was suspected.
History of past illness
The child was born from the first healthy pregnancy and delivered on time. In early childhood, he was prone to recurrent respiratory tract infections that frequently led to bronchial obstruction. He was regularly monitored by a pediatrician specializing in allergic diseases and when needed treated with bronchodilators (salbutamol), corticosteroids (budesonid) and leukotrien receptor antagonist (montelukast). Last allergy testing, done a year ago, showed slightly elevated levels of total IgE (IgE=315 kU/L) and Eosinophil Cationic Protein (ECP=41.80 μg/L).
At the present time, he is stable, on the long term Flixotide inhaler (containing 125 mg of fluticasone propionate in each puff) and Melarth (montelukast) therapy.
Family history
His parents are in good health and deny any serious illnesses in the family.
Physical examination
On admission he had a Body Mass Index (BMI) 14.4, which, with a height of 103 cm and weight of 15.3 kg, falls into a normal growth curve. However, Standard Deviation Score (SDS) was 1.15 which is below the average score for his age. His vital signs were stable and within the normal range, body temperature was 36.8°C, heart rate 80/min and blood pressure was 102/75 mmHg; he was conscious, eupnoic, properly hydrated with clean fair skin, discretely rougher perianally. His abdomen was slightly distended but without pain or organomegaly and with normal peristalsis.
Laboratory examinations
On admission, blood samples were collected for hematological and biochemical analysis.
The results of laboratory testing were unremarkable including Complete Blood Count test (CBC), Differential Blood Count test (DBC), C-reactive protein (CRP) level (11.3 mg/L), additional, metabolic panel (CMP) as well as antibodies on tissue transglutaminase (anti-tTg IgA). However, the patient had high levels of fecal calprotectin (1599 μg/g).
During hospitalization these diagnostic tests were repeated several times with the same results; even the value of fecal calprotectin significantly decreased (58 μg/g).
Additional diagnostic procedures, including abdominal ultrasound, skin prick test on nutritional allergies and testing for occult blood in stool, were made. All of these tests were negative.
Stool samples were taken for routine diagnostics of ova and parasites (Scotch tape test and microscopic examination of concentrated stool samples), rapid Enzime Immuno Assay (EIA) for gastrointestinal viruses (Rotavirus, Adenovirus and Norovirus), Helicobacter pylori antigen and for Clostridioides difficile, as well as for routine cultivation of the most prevalent enteropathogens (Salmonella, Shigella, enteropathogenic Escherichia coli and Campylobacter). For bacterial cultivation, Salmonella-Shigella agar (SS agar) under aerobic conditions on 36°C and Karmali agar (Biolife, Italy) at 42°C under microaerophilic conditions (Genbox, bioMérieux, France) were used.
All rapid EIA tests, as well as test for gastrointestinal parasites (GIT) were negative. On SS agar, only normal microbiata was cultivated.
After 48 hours of incubation on Karmali agar, small, round, light brown colonies, highly suspected to Campylobacter, were grown. Unexpectedly, colonies were identified as Aliarcobacter butzleri using Matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry (MALDI-TOF MS, Bruker, Germany) with score 2.18.
Since there is no standardised method for antimicrobial susceptibility testing of A. butzleri, testing was performed on MH-F medium according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) protocol for Campylobacter spp. [12]. Strain was susceptible in standard dosing regimen (S) to tetracycline (zone diameter: 23 mm), while susceptible in increased exposure (I) to ciprofloxacin (27 mm). Zone diameter for erythromycin was 20 mm, so according to criteria for C. jejuni, strain was susceptible, while according to criteria for C. coli, the strain was resistant to macrolides.
Treatment and outcome
During the hospitalization, appart from his chronic montelucast therapy, the boy was properly hydrated and treated only with probiotic and enteral nutrition. The diarrhea resolved exclusively with those symptomatic measures thus suggesting a self-limited course of disease.
The boy was discharged in a good clinical condition, except a mild degree of malnutrition, for which a polymeric preparation (PediaSure) was introduced in his diet.
Based on the clinical presentation, results of all diagnostic procedures and the course of disease, it was concluded that the patient had a post-infectious gastrointestinal motility disorder, followed by an acute but self-limited infection caused by the Campylobacter-like bacterium Aliarcobacter butzleri.
Isolation of A. butzleri, in the absence of other enteropathogenic bacteria, parasites and enteroviruses, along with self-limited remission of the patient’s symptoms, strongly suggest that in this specific case, A. butzleri was the etiological agent. To the best of our knowledge, this is the first reported human case of acute watery diarrhea due to A. butzleri in Croatia.
Literature review
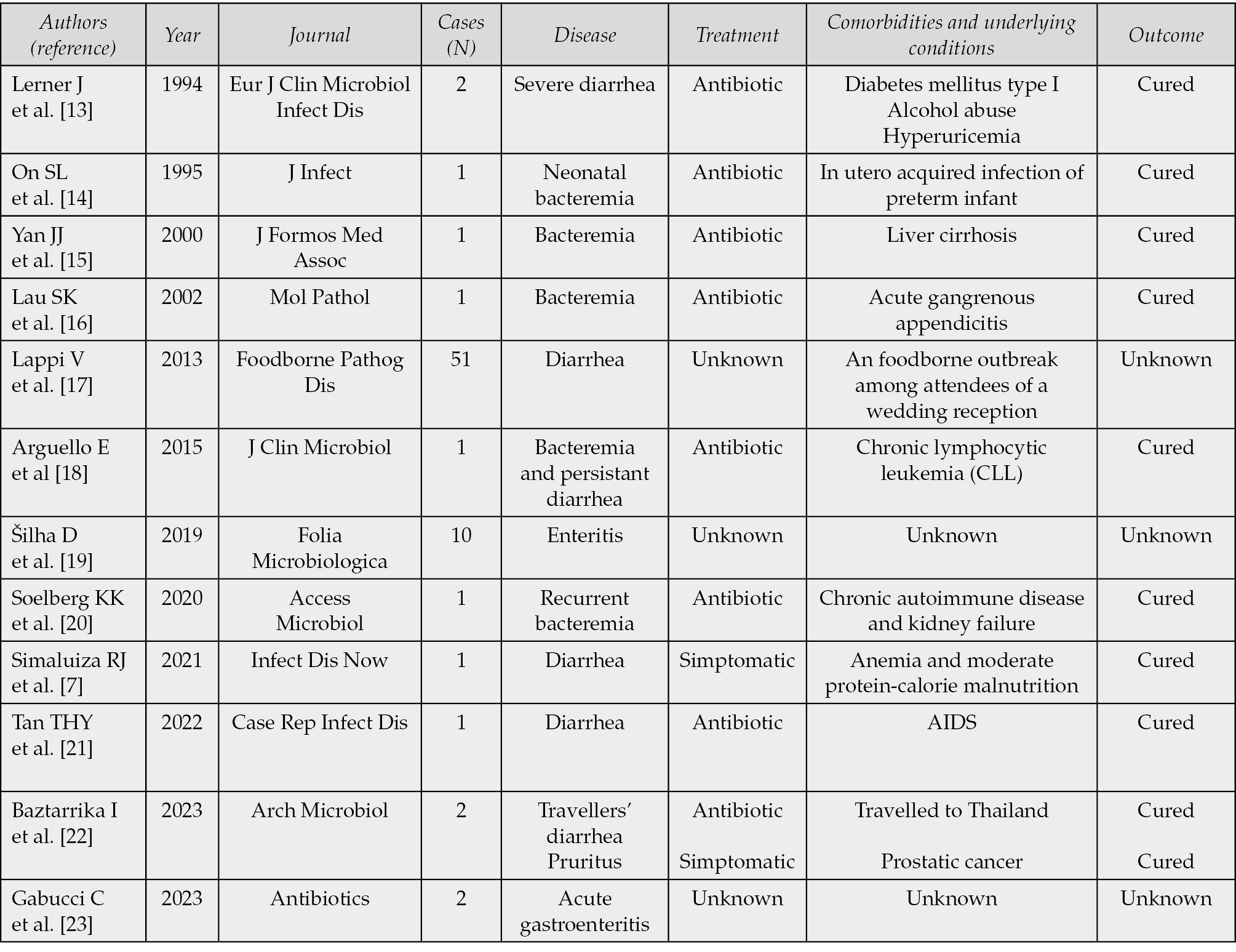
We performed aslo a literature search in PubMed/MEDLINE and Google Scholar database on 1st May 2024 using the terms “Aliarcobacter butzleri” AND “clinical case”. A total of 149 research papers published in 30-years period, from 1993 to 2024 (15 in PubMed and 134 in Google Scholar) were identified and screened. Among them, we selected, by abstract or full text reading, 10 articles written in English (case reports and case series), whose main characteristics have been summarized in Table 1.
We excluded any article that didn’t include at least one case of human infection caused by Aliarcobacter butzleri and cases caused with other Aliarcobacter species.
Table 1 - Case reports identified by literature search.
DISCUSSION
Over the last few years, A. butzleri has been recognized as an emerging foodborne and waterborne pathogen that can cause prolonged watery diarrhea in young children. Aliarcobacteriosis may be presented with abdominal pain, cramps, severe diarrhea and fever. A. butzleri has worldwide distribution and human aliarcobacteriosis has been already documented in many European countries (e.g. Italy, Spain, France, United Kingdom, Belgium and Germany), Asia, South Africa and Latin America [5, 6].
However, due to the lack of standardized cultivation and identification methods, as well as the lack of awareness of the public health significance, many infections remain unrecognized and etiologically unproven [4, 5, 24].
A. butzleri is a part of the intestinal microbiota of many healthy animals, so it can be isolated not only from diarrheic stool, but also from normal fecal (e.g. chicken, sheep, pigs and cattle) samples. Therefore, it is considered as an opportunistic pathogen in veterinary medicine. It can be transmitted vertically and horizontally between the animals, and the global prevalence in animal stool samples range from 0.8% in Italy to 100% in Turkey [1].
Human infections usually follow ingestion of fecally contaminated food or water. Food contamination may occur at any step in food supply chain and usually occurs from poor and insufficient hygiene. Contaminated raw milk and undercooked poultry meat are the most prevalent sources for human infection [6]. Besides, A. butzleri has been isolated from various water samples, like river water, seawater and especially groundwater and wastewater (e.g. in Spain, Italy, UK and USA). Moreover, it may survive in non-chlorinated drinking water, in wide temperature range, for up to 16 days [6].
Microbiological detection of A. butzleri is challenging, since several issues in cultivation and identification have arisen. Different selective and non-selective media are used for the isolation of A. butzleri, and the composition of the nutrient media itself can significantly affect the percentage of successful cultivation [25]. Moreover, identification at the species level is unreliable with classical biochemical and phenotypic tests, since they don’t have sufficient discriminatory power [6]. However, modern rapid and specific diagnostic methods and techniques, such as PCR assays, are increasingly used in clinical microbiology laboratories [6]. Webb et al. have found that the rate of detection of A. butzleri in human stools by isolation was low (0.8%) compared to the rate of PCR-based detection (60%) [9].
Besides, little is known about susceptibility and resistance of A. butzleri to the antibiotics that are usually given in the treatment of severe forms of bacterial diarrhea. Due to the lack of specific guidelines and specific breakpoints for Aliarcobacter, different susceptibility testing techniques (disk diffusion, agar plate dilution, broth microdilution, gradient strip diffusion, semiautomated methods) and different criteria are used [1, 6].
Until specific breakpoints are established, authors use CLSI (Clinical Laboratory Standards Institute) for Enterobacteriaceae, Staphyloccous and Campylobacter, or European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for Enterobacteriaceae, Campylobacter and non-species-related breakpoints. Therefore, it’s very hard to compare results from different researches and studies [1].
Aliarcobacteriosis is usually a self-limited disease and antibiotics are recommended only for severe clinical course. Since Aliarcobacter is closely related to Campylobacter, fluoroquinolones and macrolides were initially thought to be the antibiotics of choice. However, there are several studies that report resistance of Aliarcobacter to these antibiotics. According to the results from Van den Abeele et al., tetracycline are considered to be effective for the treatment of fluoroquinolone-resistant isolates, which is in concordance with the results obtained in the study from de Oliveira et al. [5, 10].
Although the pathogenesis of aliarcobacteriosis has not been fully elucidated, A. butzleri can trigger the onset of an inflammatory reaction of the intestinal crypts, with thickening and flattening of villi, tissue destruction and infiltrations with macrophages, lymphocytes and heterophils. Moreover, biofilm formation and positive toxicity test have been demonstrated in Verro and HeLa cells [5, 13].
After an acute bacterial infection caused by A. butzleri, time is needed for the intestinal mucosa recovery and normal resorption of nutrients. Therefore, prolonged watery diarrhea is not a surprising outcome of the infection. A diet that includes easily digestible and nutrient-rich foods and preparations with sufficient fluid intake is recommended, until the normal physiological process of recovery of the intestinal mucosa is completed [7].
CONCLUSIONS
To the best of our knowledge, this is the first documented human case of acute watery diarrhea due to Aliarcobacter butzleri in Croatia.
We have isolated Aliarcobacter butzleri during the routine stool cultivation on selective media for Campylobacter. Grown colonies of A. butzleri looked like Campylobacter and if we didn’t use MALDI-TOF, we would probably misidentify the strain as Campylobacter.
Our report highlights the need to establish standardized protocols to optimize diagnostic and identification methods in order to minimize misdiagnosis and provide comparable and reliable data establishing the prevalence and significance of Alaicobacter infections.
Conflict of interest
None.
Funding
No funding for this paper was received.
Authors’ contribution
MVT and AN: conceptualization, investigation, writing, revising and editing. VŽ and MT: investigation and revising the manuscript. AN and MVT contributed equally.
REFERENCES
[1] Çelik C, Pınar O, Sipahi N. The Prevalence of Aliarcobacter Species in the Fecal Microbiota of Farm Animals and Potential Effective Agents for Their Treatment: A Review of the Past Decade. Microorganisms. 2022; 10(12): 2430. doi: 10.3390/microorganisms10122430. Accessed 11 March 2024.
[2] Vandenberg O, Dediste A, Houf K, et al. Arcobacter species in humans. Emerg Infect Dis. 2004; 10(10): 1863-7. doi: 10.3201/eid1010.040241. Accessed 11 March 2024.
[3] Pérez-Cataluña A, Salas-Massó N, Diéguez AL, et al. Corrigendum (2): Revisiting the Taxonomy of the Genus Arcobacter: Getting Order From the Chaos. Front Microbiol. 2019; 10: 2253. doi: 10.3389/fmicb.2019.02253. Erratum for: Front Microbiol. 2018; 9: 2077. PMID: 31611866; PMCID: PMC6779803. Accessed 11 March 2024.
[4] Müller E, Abdel-Glil MY, Hotzel H, Hänel I, Tomaso H. Aliarcobacter butzleri from water poultry: Insights into antimicrobial resistance, virulence and heavy metal resistance. Genes. 2020; 11: 1104; doi:10.3390/genes11091104 Accessed 11 March 2024.
[5] Oliveira MGXd, Cunha MPV, Moreno LZ, et al. Antimicrobial Resistance and Pathogenicity of Aliarcobacter butzleri Isolated from Poultry Meat. Antibiotics. 2023; 12(2): 282 https://doi.org/10.3390/antibiotics12020282 Accessed 11 March 2024.
[6] Ghaju Shrestha R, Tanaka Y, Haramoto E. A Review on the Prevalence of Arcobacter in Aquatic Environments. Water. 2022; 14(8): 1266. https://doi.org/10.3390/w14081266 Accessed 11 March 2024.
[7] Simaluiza RJ, Ambuludi DR, Fernández H. First case of diarrhea due to Aliarcobacter butzleri (formerly Arcobacter butzleri) in Ecuador. Infect Dis Now. 2021; 51(6): 564-566. doi: 10.1016/j.idnow.2020.12.002. Accessed 11 March 2024.
[8] Merga JY, Leatherbarrow AJ, Winstanley C, et al. Comparison of Arcobacter isolation methods, and diversity of Arcobacter spp. in Cheshire, United Kingdom. Appl Environ Microbiol. 2011; 77(5): 1646-1650. doi: 10.1128/AEM.01964-10. Accessed 11 March 2024.
[9] Webb AL, Boras VF, Kruczkiewicz P, et al. Comparative Detection and Quantification of Arcobacter butzleri in Stools from Diarrheic and Nondiarrheic People in Southwestern Alberta, Canada. J Clin Microbiol. 2016; 54(4): 1082-1088. doi: 10.1128/JCM.03202-15 Accessed 11 March 2024.
[10] Van den Abeele AM, Vogelaers D, Vanlaere E, Houf K. Antimicrobial susceptibility testing of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from Belgian patients. J Antimicrob Chemother. 2016; 71(5): 1241-1244. doi: 10.1093/jac/dkv483. Accessed 11 March 2024.
[11] Son I, Englen MD, Berrang ME, Fedorka-Cray PJ, Harrison MA. Antimicrobial resistance of Arcobacter and Campylobacter from broiler carcasses. Int J Antimicrob Agents. 2007; 29(4): 451-455. doi: 10.1016/j.ijantimicag.2006.10.016.
[12] The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0, 2023. 2023. Available at: http://www.eucast.org/clinical_breakpoints/. Accessed 11 March 2024.
[13] Lerner J, Brumberger V, Preac-Mursic V. Severe diarrhea associated with Arcobacter butzleri. Eur J Clin Microbiol Infect Dis. 1994; 1 3(8): 660-662. doi: 10.1007/BF01973994.
[14] On SL, Stacey A, Smyth J. Isolation of Arcobacter butzleri from a neonate with bacteraemia. J Infect. 1995; 31(3): 225-227. doi: 10.1016/s0163-4453(95)80031-x.
[15] Yan JJ, Ko WC, Huang AH, Chen HM, Jin YT, Wu JJ. Arcobacter butzleri bacteremia in a patient with liver cirrhosis. J Formos Med Assoc. 2000; 99(2): 166-169.
[16] Lau SK, Woo PC, Teng JL, Leung KW, Yuen KY. Identification by 16S ribosomal RNA gene sequencing of Arcobacter butzleri bacteraemia in a patient with acute gangrenous appendicitis. Mol Pathol. 2002; 55(3): 182-5. doi: 10.1136/mp.55.3.182.
[17] Lappi V, Archer JR, Cebelinski E, et al. An outbreak of foodborne illness among attendees of a wedding reception in Wisconsin likely caused by Arcobacter butzleri. Foodborne Pathog Dis. 2013; 10(3): 250-255. doi: 10.1089/fpd.2012.1307.
[18] Arguello E, Otto CC, Mead P, Babady NE. Bacteremia caused by Arcobacter butzleri in an immunocompromised host. J Clin Microbiol. 2015; 53(4): 1448-1451. doi: 10.1128/JCM.03450-14.
[19] Šilha D, Vacková B, Šilhová L. Occurrence of virulence-associated genes in Arcobacter butzleri and Arcobacter cryaerophilus isolates from foodstuff, water, and clinical samples within the Czech Republic. Folia Microbiol. 2019; 64(1): 25-31. doi: 10.1007/s12223-018-0628-x.
[20] Soelberg KK, Danielsen TKL, Martin-Iguacel R, Justesen US. Arcobacter butzleri is an opportunistic pathogen: recurrent bacteraemia in an immunocompromised patient without diarrhoea. Access Microbiol. 2020; 12: 2(8): acmi000145. doi: 10.1099/acmi.0.000145.
[21] Tan THY, Tham SM, Tambyah PA. Arcobacter butzleri in an AIDS patient. Case Rep Infect Dis. 2022; 2022: 6983094. doi: 10.1155/2022/6983094.
[22] Baztarrika I, Salazar-Sánchez A, Hernaez Crespo S, et al. Virulence genotype and phenotype of two clinical isolates of Arcobacter butzleri obtained from patients with different pathologies. Arch Microbiol. 2023; 205(12): 369. doi: 10.1007/s00203-023-03709-3.
[23] Gabucci C, Baldelli G, Amagliani G, et al. Widespread multidrug resistance of Arcobacter butzleri isolated from clinical and food sources in central Italy. Antibiotics (Basel). 2023; 12(8): 1292. doi: 10.3390/antibiotics12081292.
[24] Douidah L, de Zutter L, Baré J, et al. Occurrence of putative virulence genes in Arcobacter species isolated from humans and animals. J Clin Microbiol. 2012; 50(3): 735-741. doi: 10.1128/JCM.05872-11.
[25] Fera MT, Gugliandolo C, Lentini V, et al. Specific detection of Arcobacter spp. in estuarine waters of Southern Italy by PCR and fluorescent in situ hybridization. Lett Appl Microbiol. 2010; 50(1): 65-70. doi: 10.1111/j.1472-765X.2009.02767.x.