Le Infezioni in Medicina, n. 2, 231-240, 2024
doi: 10.53854/liim-3202-11
CASE REPORTS
Dalbavancin for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in pediatric patients: a case series
Valeria Garbo1,2, Anna Condemi1,2, Chiara Albano1, Valentina Frasca Polara2, Roberta Parrino2, Alessandra Macaluso2, Laura Venuti1, Claudia Colomba1,2
1Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties “G. D’Alessandro”, University of Palermo, Palermo, Italy;
2Division of Paediatric Infectious Disease, “G. Di Cristina” Hospital, ARNAS Civico Di Cristina Benfratelli, Palermo, Italy
Article received 18 April 2024, accepted 7 May 2024
Corresponding author
Claudia Colomba
E-mail: claudia.colomba@libero.it
SummaRY
Acute bacterial skin and skin-structure infections (ABSSSI) are a significant cause of morbidity in pediatric patients, requiring timely and effective treatment. Dalbavancin, a long-acting lipoglycopeptide antibiotic recently approved for pediatric use, offers advantages such as excellent bactericidal activity against Gram-positive bacteria (including multidrug-resistant pathogens) and high tissue penetration.
We present a case series of pediatric patients with ABSSSI treated with dalbavancin. Five cases were described demonstrating the efficacy of dalbavancin in different clinical scenarios. Patients with complex skin conditions, including cellulitis and deep abscesses, benefited from dalbavancin therapy, achieving significant clinical improvement. Notably, dalbavancin facilitated early discharge, improving quality of life and reducing healthcare costs.
These cases highlight the potential of dalbavancin as a valuable treatment option for ABSSSI in pediatric patients, particularly in settings where conventional therapies fail to achieve optimal clinical outcomes or prolonged hospitalization is not feasible.
Further research is needed to clarify its role and optimize its use in pediatric patients with ABSSSI.
Keywords: Dalbavancin; acute bacterial skin and skin structure infections (ABSSSIs); cellulitis; skin abscesses; pediatric patients.
INTRODUCTION
Acute bacterial skin and skin-structure infections (ABSSSIs) are a subgroup of complicated skin and soft tissue infections (SSTIs) and are a frequent cause of morbidity in both the community and the hospital setting [1-3]. ABSSSIs have been defined in 2013 by the US Food and Drug Administration (FDA), and include cellulitis, erysipelas, major skin abscesses, and wound infections, with a lesion size of at least 75 cm2 measured by the area of redness, edema, or induration [4]. Although the definition of complicated abscess in children often considers parameters like age-specific maximum diameter, the number of lesions, and potential recurrence, there is currently no established specific size cutoff for ABSSSIs in the pediatric population.
The most common etiological agents of ABSSSI are Staphylococcus aureus, including methicillin-resistant (MRSA) strains, and Streptococcus pyogenes. Less commonly, some infections can involve other Streptococci, enterococci, and Gram-negative bacteria [5].
ABSSSIs are a significant source of morbidity in children, with skin abscesses and cellulitis being the predominant skin infections treated by pediatricians [6, 7]. In the United States, ABSSSIs result in approximately 3 million pediatric health care visits per year, placing a heavy burden on the healthcare system [8]. The increasing occurrence of community-associated MRSA has caused hospitalizations for ABSSSI in children to double between 1997 and 2009, exceeding 70,000 per year [9]. If diagnosed early and treated appropriately, these infections are almost always curable, but some can potentially cause prolonged hospitalization and life-threatening complications [10, 11].
Dalbavancin is a long-acting, semi-synthetic lipoglycopeptide antibiotic with a unique pharmacokinetic profile, characterized by a long half-life (lasting about two weeks in adults), excellent bactericidal activity against Gram-positive bacteria (including multidrug-resistant pathogens), a good safety profile, and high tissue penetration [12]. Although most of the evidence comes from studies conducted on adults, the innovative mode of action of dalbavancin and the consequent advantages have made it an ideal candidate for the treatment of ABSSSI even in the pediatric setting. In fact, in December 2022, the EMA approved the extension of dalbavancin for the treatment of pediatric patients with ABSSSI [13]. For children, the dose of dalbavancin depends on age and body weight and should be no more than 1500 mg. Specifically, the recommended dose for infants and children aged 3 months to less than 6 years is a single dose of 22.5 mg/kg, and for children and adolescents aged 6 years to less than 18 years the recommended dose is a single dose of 18 mg/kg [14, 15].
The aim of this paper was to collect and report the current experience on the use of dalbavancin in pediatric patients admitted to the Department of Pediatric Infectious Diseases at “G. Di Cristina” Hospital in Palermo from January to December 2023.
CASE PRESENTATIONS
Case 1
A 13-year-old female patient affected by Clericuzio-type Poikiloderma with neutropenia syndrome under treatment with Granulocyte-Colony Stimulating Factor (G-CSF), was admitted to the hospital for 5-day onset of fever (maximum body temperature [TCmax], 39 °C) and erythematous-nodular lesions. These lesions were described as hard, infiltrated, painful, and warm to the touch, distributed across the entire skin surface but prevalent in the lower limbs, consistent with the diagnosis of cellulitis (Figure 1).
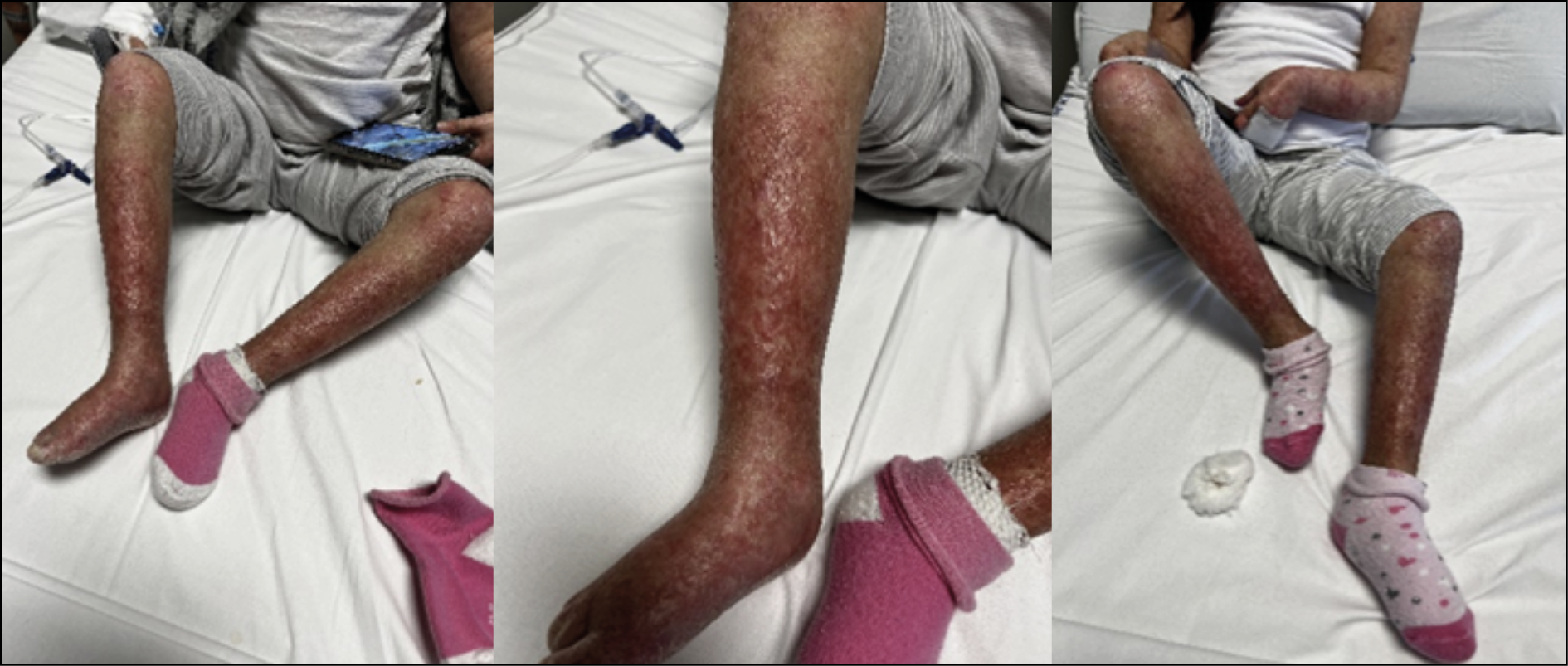
Figure 1 - Skin lesions on the lower limbs, indicative of cellulitis, observed in the patient on admission.
Blood tests revealed mild leukopenia (White Blood Cells [WBC]: 2,900/uL) associated with elevated C-reactive protein (CRP) (13.55 mg/dl). All blood cultures and serological investigations were negative.
Because of the patient’s frequent hospitalizations and susceptibility to infectious complications due to the underlying disease, empirical broad-spectrum antibiotic therapy with daptomycin (10 mg/kg/day) and meropenem (60 mg/kg/day in three administrations) was initiated. However, after six days of treatment, the clinical response was suboptimal. Although the patient presented with lower febrile episodes and spaced peaks over 24 hours, no improvement in the skin lesions on the limbs was appreciated.
Given the poor clinical response to antibiotic therapy and the patient’s poor compliance in accepting a long hospital stay, a single dose of intravenous dalbavancin (18 mg/kg) was administered on the eighth day of hospital stay, and the patient was discharged after 24 hours. At follow-up seven days later, significant improvement of skin lesions was noted (Figure 2).
At the second and final follow-up, three weeks after discharge, complete healing of the skin lesions was observed. However, the child, affected by a rare genetic syndrome characterized primarily by poikiloderma and telangiectasias, frequently experiences skin ulceration (last hospitalization in March 2024).
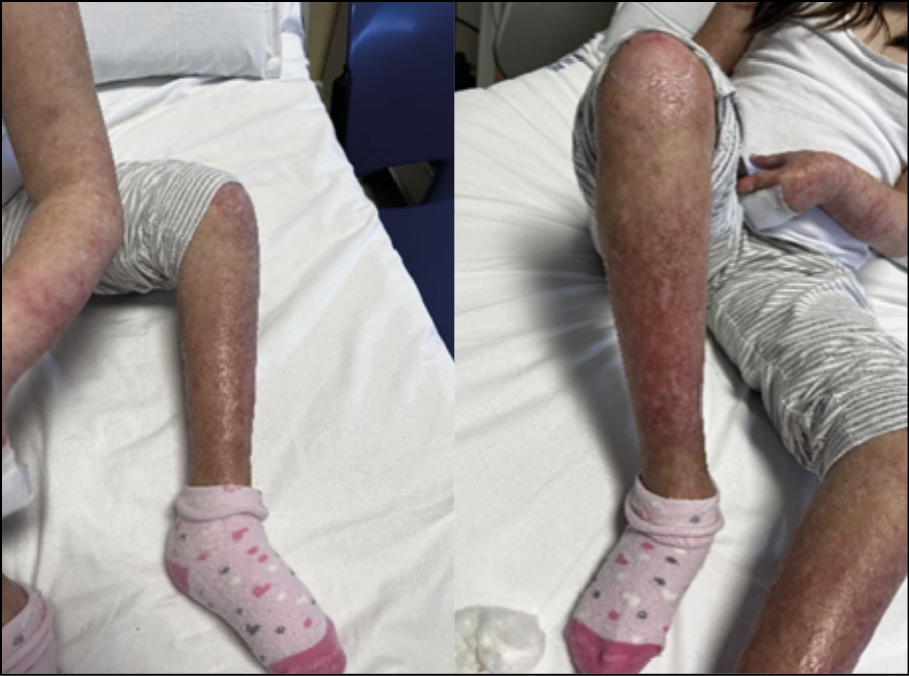
Figure 2 - Skin lesions significantly improved at follow-up seven days after discharge following a single dose of dalbavancin.
Case 2
A 12-year-old girl presented to the emergency room with fever, persistent headache for several days, and bilateral eyelid edema. Despite previous antibiotic therapy with macrolides, there was little benefit.
Upon admission to the Department of Pediatric Infectious Diseases, the patient appeared in fair clinical condition, with fever and normal cardio-respiratory activity. She presented with bilateral periorbital edema associated with conjunctival hyperemia, with preserved ocular motility and pupillary reflex (Figure 3).

Figure 3 - Bilateral periorbital edema associated with conjunctival hyperemia observed in the patient upon admission.
Blood tests revealed marked leukocytosis with neutrophilia (WBC: 38,930/uL, Neutrophils: 34,270/uL) and an elevated CRP (23.17 mg/dl). Blood cultures were negative. Cranial computed tomography (CT) scan and brain Magnetic Resonance Imaging (MRI) revealed bilateral periorbital cellulitis associated with pansinusitis (Figure 4). Therefore, endoscopic drainage of the paranasal sinuses was performed, and from the culture examination of the drained material, S. pneumoniae was isolated.
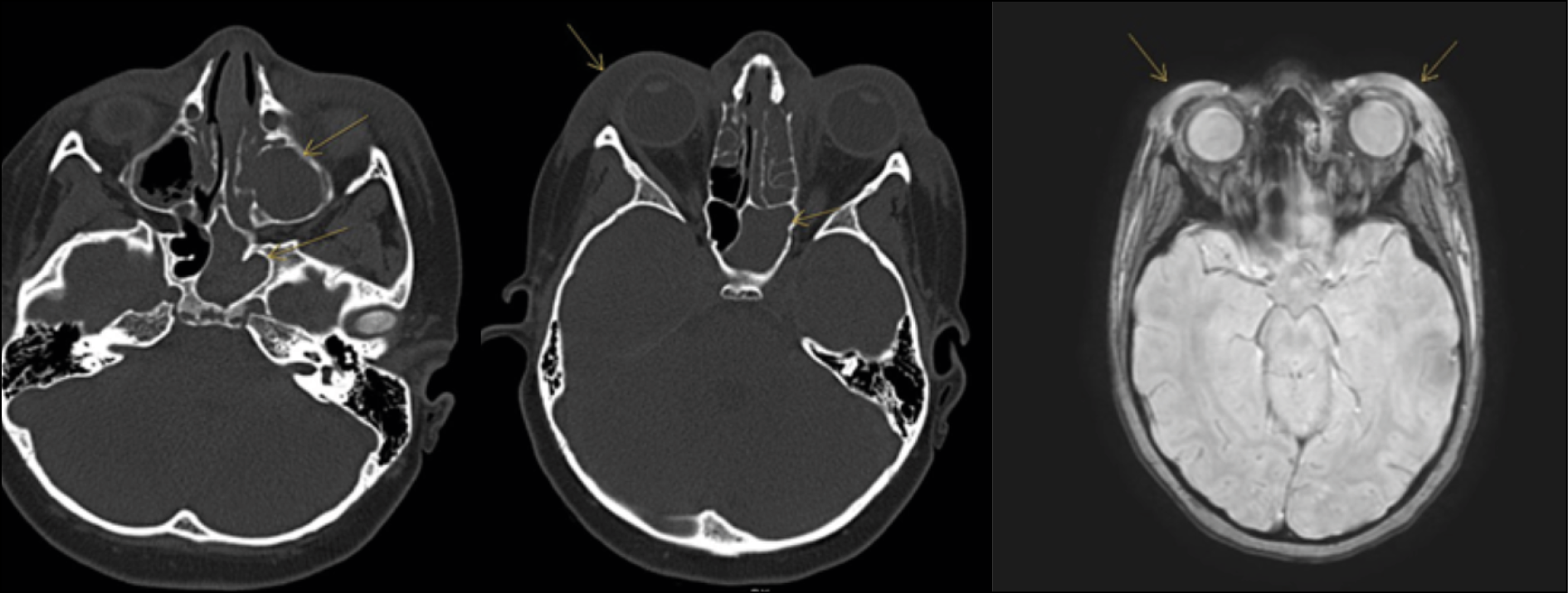
Figure 4 - a) Cranial CT scan: repletion of ethmoid sinuses or ethmoid air cells; b) Cranial CT scan: Periorbital tissue edema associated with cellulitis; c) Brain MRI: periorbital tissue edema.
The patient received antibiotic therapy with ceftriaxone (100 mg/kg/day) and metronidazole (30 mg/kg/day in three administrations), resulting in partial clinical improvement. However, due to family needs, voluntary discharge was requested after five days of treatment.
To ensure adequate antibiotic coverage, given the context of poor family compliance, a single dose of dalbavancin (18 mg/kg) was administered 24 hours before discharge, following a 7-day hospital stay. At follow-up 72 hours after discharge, the patient showed significant clinical improvement, with resolution of fever and a marked reduction of periorbital edema (Figure 5). The patient fully recovered, and there were no subsequent recurrences.
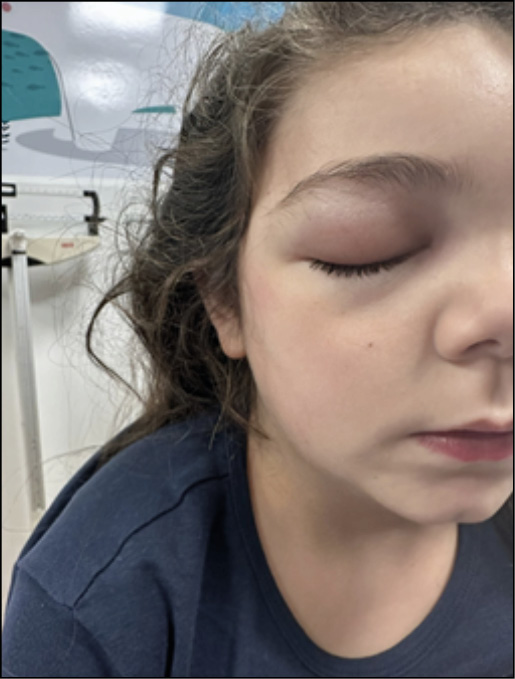
Figure 5 - Marked reduction of periorbital edema observed at follow-up 72 hours after discharge following a single dose of dalbavancin.
Case 3
A 15-month-old girl was admitted to the Department of Pediatric Infectious Diseases due to the onset of fever and a painful, infiltrated erythematous lesion on the anterolateral surface of the right thigh, which appeared 24 hours after receiving the third dose of hexavalent vaccine.
On admission, the child was febrile, agitated, and presented with cellulitis involving the skin of the right thigh, characterized by edema, erythema, warmth, and tenderness to palpation (Figure 6).
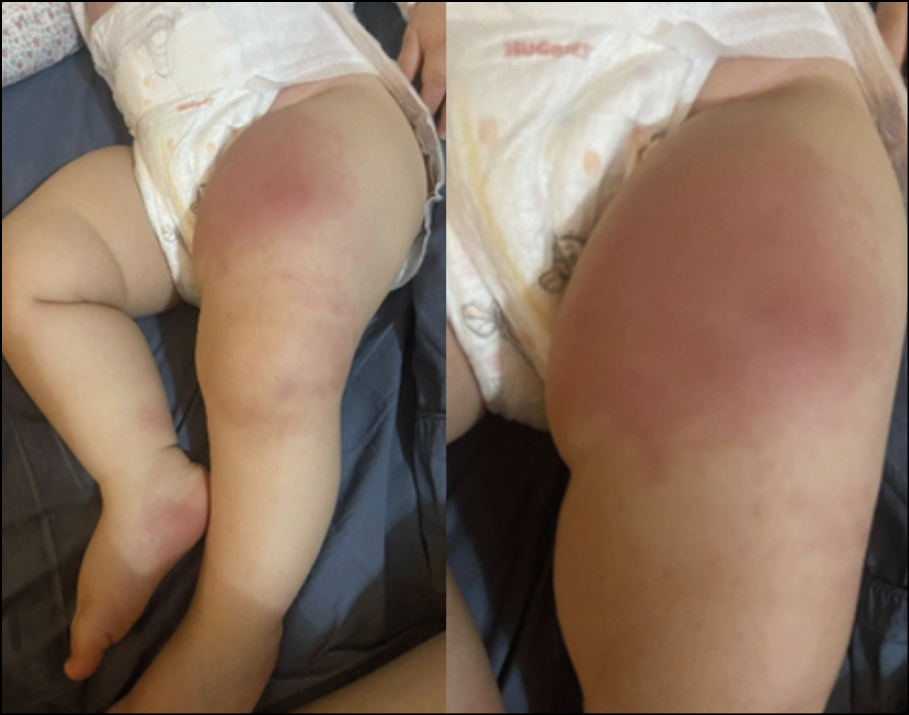
Figure 6 - Cellulitis involving the skin of the right thigh observed in the neonate upon admission.
Blood tests showed neutrophilic leukocytosis (WBC: 24,740/uL, Neutrophils: 72%) and elevated CRP (3.3 mg/dl), and blood cultures were negative. Ultrasound of the subcutaneous tissue revealed a large fluid collection above the quadriceps muscle, consistent with an abscess.
The patient received intravenous daptomycin therapy (10 mg/kg/day) for 7 days with partial clinical benefit. To facilitate a shorter hospital stay, antibiotic therapy was switched to dalbavancin (22.5 mg/kg single dose), and the patient was discharged after 24 hours, following a 10-day hospital stay.
At follow-up 72 hours after discharge, the patient showed a marked reduction in the extent of the erythematous area and edema of the thigh, which was no longer painful. However, she presented with low-grade fever and a diffuse maculo-papular exanthema all over the skin, mainly affecting the limbs, related to an Adenovirus infection acquired during hospitalization (Figure 7). At the second outpatient visit, 10 days after discharge, the patient no longer had any signs of inflammation.
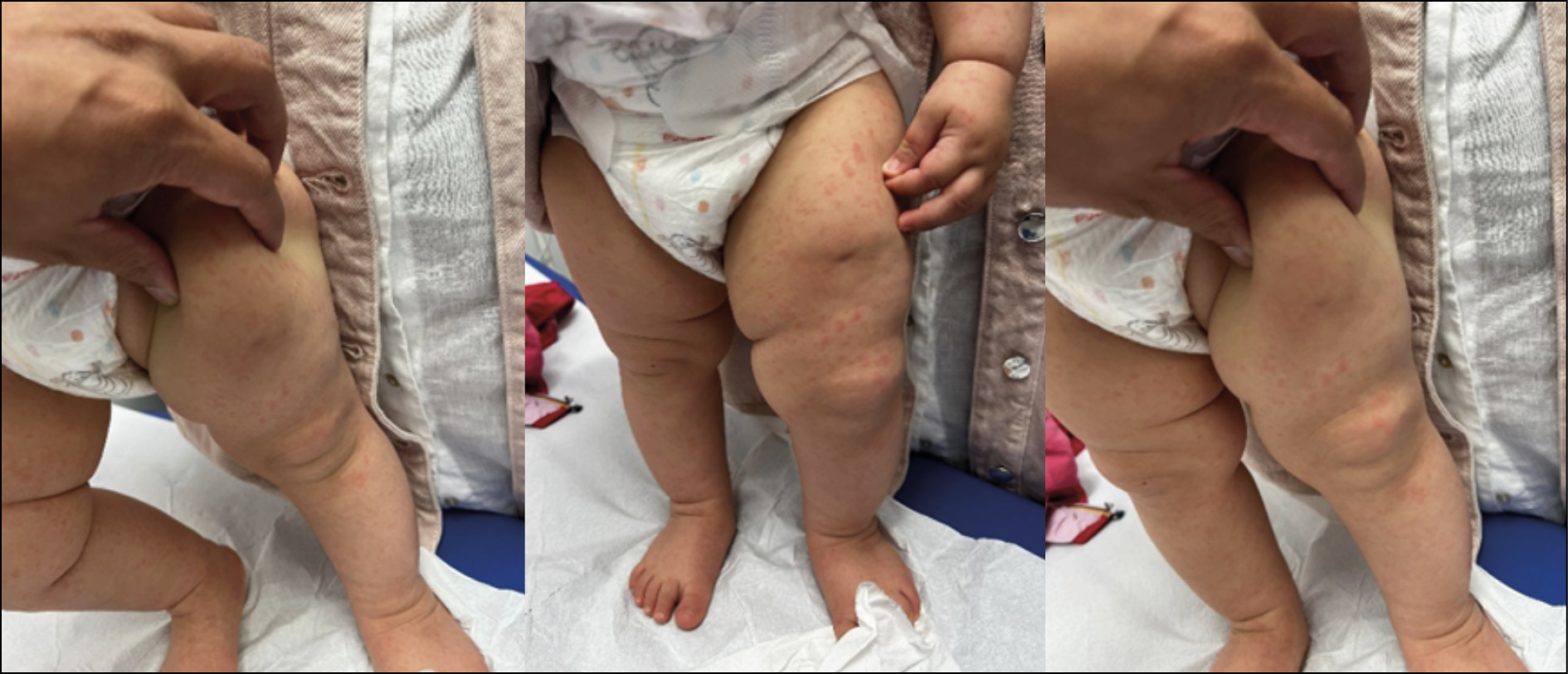
Figure 7 - Diffuse maculo-papular exanthema covering the entire skin surface, predominantly affecting the limbs, related to an Adenovirus infection acquired during hospitalization.
Case 4
A previously healthy 10-year-old boy presented to the emergency room with left knee pain after a soccer game. Initial X-rays ruled out fractures, and the limb was immobilized with a plaster splint before discharge. However, two days later, due to the appearance of fever, chest pain and tachypnea, the child accessed again the emergency room and was later transferred to the Department of Pediatric Infectious Diseases. Upon admission, the patient appeared in pain, and exhibited fever and tachypnea (respiratory rate of 65 breaths per minute).
Physical examination revealed a reduced vesicular murmur at the base of the right lung and hypophony on the left lung, as well as abdominal tenderness. The plaster splint immobilizing the left lower limb was removed, revealing heat, soreness, and hypomobility.
Blood tests showed neutrophilic leukocytosis (WBC: 13,240/ul, Neutrophils: 12,220/ul) associated with an increased CRP (52.77 mg/dl).
Further investigations, including chest CT with contrast medium and lower extremity echocolor-doppler, documented severe bilateral necrotizing pneumonia and sub-occlusive thrombosis of multiple veins, respectively. Blood cultures and broncho-alveolar lavage isolated Methicillin-Resistant S. aureus (MRSA).
Based on the antibiogram results, antibiotic therapy with linezolid (20 mg/kg/day divided into two doses) and ceftaroline (12 mg/kg/8 h), along with anticoagulant therapy with heparin, was initiated.
On the sixth day of hospitalization, a MRI scan of the left lower limb revealed arthritis of the knee joint and extensive supra- and subfascial femoral abscess collections.
However, due to the severity of the clinical presentation and identification of genes associated with Panton-Valentine toxin production and cefotixin resistance, antibiotic therapy was modified to fosfomycin (400 mg/kg/day administered in three doses), clindamycin (40 mg/kg/day in three administrations), and daptomycin (7 mg/kg/day).
After 4 weeks of antibiotic therapy, the patient clinically improved, no longer presenting with fever or chest pain; however, pain and limited mobility persisted in the left lower limb.
A follow-up chest CT scan revealed significant improvement in lung condition, while MRI findings of the femur and left knee raised concern. Therefore, a bone biopsy was performed at a specialized orthopedic institute, after eight weeks of therapy. Pending biopsy results, the patient was discharged 24 hours after receiving a single dose of dalbavancin (18 mg/kg), following an 8-week hospitalization period.
Approximately two months after discharge, follow-up MRI described marked reduction of perifemoral abscess collections, the presence of concentric lamellar calcifications of the femoral diaphysis, and rarefaction of the bone cortical of the lateral femoral condyle. Echocolor-doppler documented resolution of the previously described thrombosis.
The patient is currently in outpatient follow-up, exhibiting general improvement.
Case 5
A previously healthy 3-month-old infant was brought to the emergency room with hyperpyrexia and edema of the left lower extremity, following meningococcal B vaccination 12 days earlier. Despite prior treatment with amoxicillin/clavulanic acid, the symptoms persisted.
Upon admission to the Department of Pediatric Infectious Diseases, the infant appeared agitated but was apyretic and had normal breathing. Physical examination revealed a flexed, externally rotated, edematous and intensely painful left lower limb with tight, warm skin, accompanied by a small pustule at the vaccine inoculation site (Figure 8).
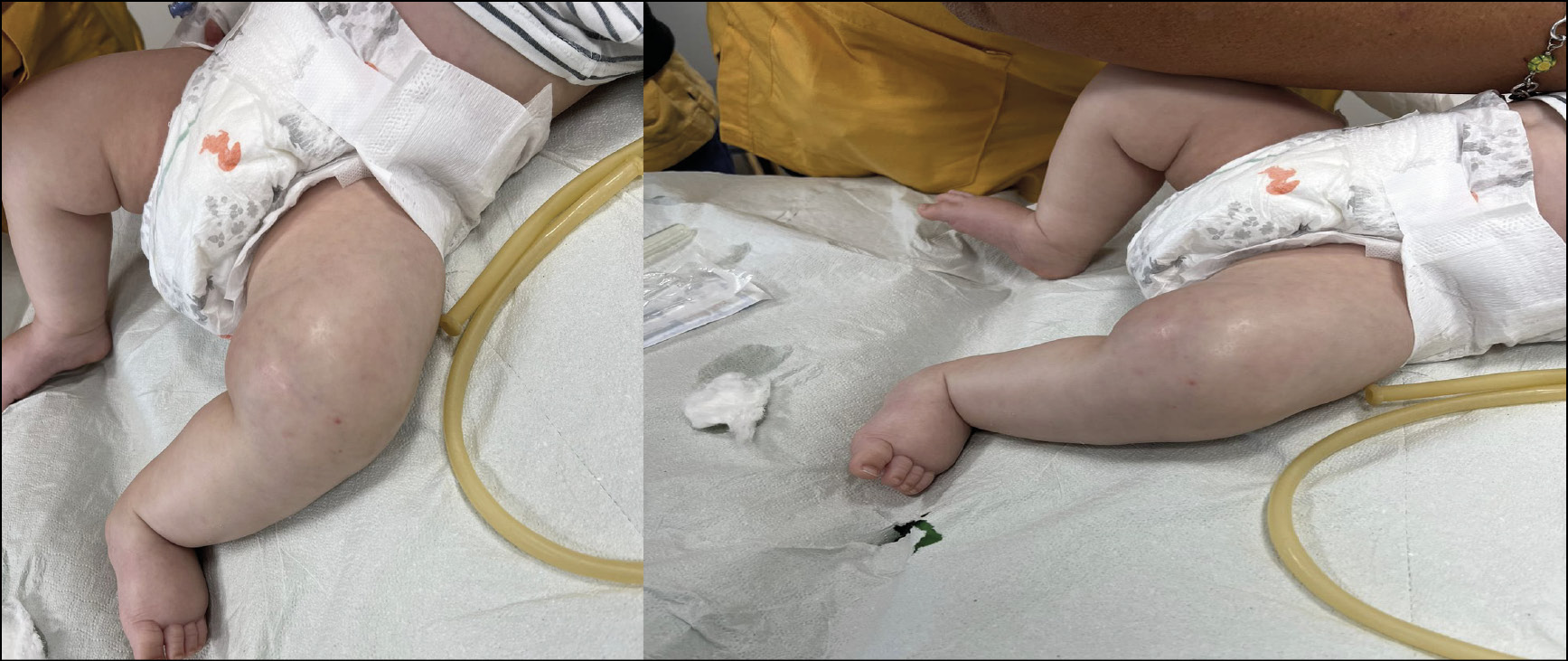
Figure 8 - Cellulitis involving the skin of the left lower limb observed in the neonate upon admission.
Blood tests indicated neutrophilic leukocytosis (WBC: 24,520/uL, Neutrophils: 16,050/uL) and elevated CRP (26.06 mg/dl). Microbiological investigations (pharyngeal swab, nasal swab for respiratory virus, blood culture, septifast) were negative, prompting empirical antibiotic therapy with meropenem (60 mg/kg/day in three administrations) and daptomycin (10 mg/kg/day).
CT scan documented the presence of cellulitis along with a large, multiloculated, capsular abscess with a total size of 9x4 cm in the perischelectric subfascial planes of the left femur, extending from the femoral neck to the distal metadiaphyseal region, associated with disconnection of the muscular plane from the bone, infiltration of the knee joint, and osteomyelitis of the femur (Figure 9).
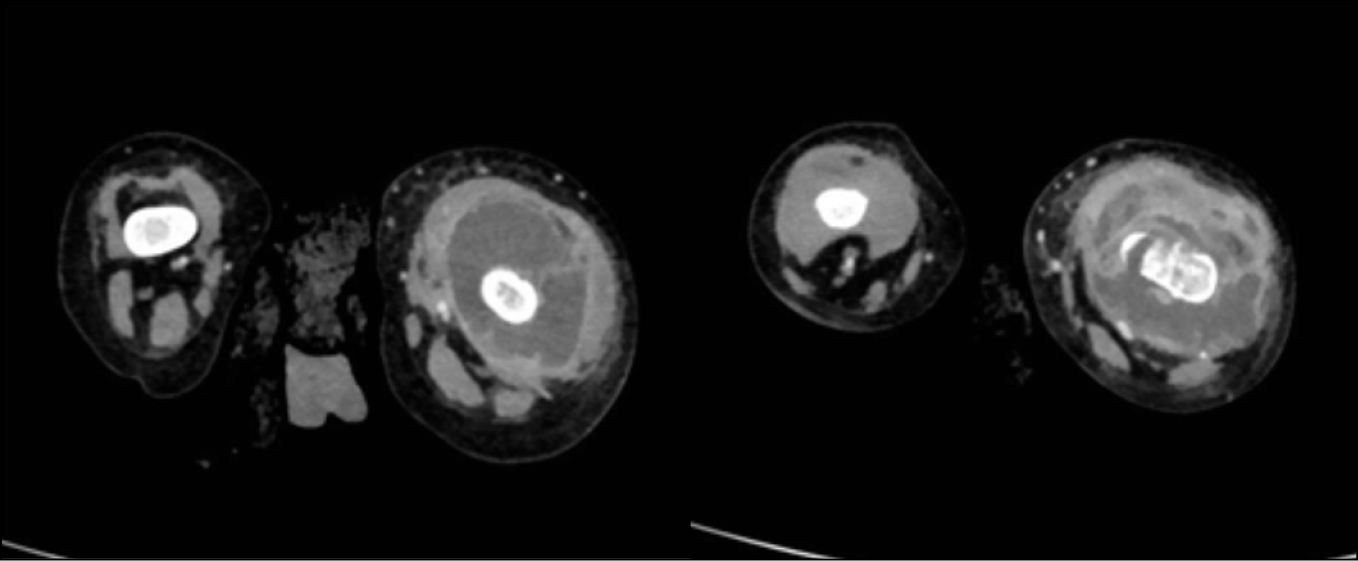
Figure 9 - CT images show cellulitis and a large, multiloculated capsular abscess measuring 9 x 4 cm in the perischelectric subfascial planes of the left femur.
Surgical aspiration of the abscess with placement of a drainage tube revealed Methicillin-Sensitive S. aureus (MSSA), likely inoculated during vaccination. Antibiotic therapy was adjusted to cefazolin (150 mg/kg/day divided into three doses) and rifampicin (10 mg/kg/day in two doses) in combination with steroid therapy for 6 weeks, resulting in partial improvement and gradual recovery of spontaneous limb movement.
A re-evaluation MRI showed a reduction in abscess thickness but marked edematous imbibition of adjacent soft tissues, periosteal reaction of the femoral diaphysis, and substantial bone remodeling of the distal metaphyseal region. Before discharge, a single dose of dalbavancin (22.5 mg/kg) was administered following a 4-week hospitalization period.
At 1-month follow-up, the patient had improved clinically, with the limb no longer appearing edematous and not painful on either palpation or active and passive movement. Ultrasound control showed further reduction in abscess thickness.
DISCUSSION
The cases presented illustrate different clinical scenarios in which dalbavancin was used in pediatric patients, demonstrating its potential role in the management of complicated infections. In settings where conventional antibiotic therapies failed to achieve optimal clinical outcomes or in cases where prolonged hospital stay was not feasible due to various factors, dalbavancin emerged as a valuable treatment option.
This study described 5 clinical cases of children with ABSSSI, with confirmed or highly suspected diagnosis of Gram-positive bacterial infection, treated with dalbavancin. Among these cases, three were diagnosed as cellulitis, while the remaining two were identified as deep abscesses.
Microbiological study provided Gram-positive bacterial isolate in 3 cases: S. pneumoniae from sinus drainage (Case 2), MRSA from positive blood cultures and broncho-alveolar lavage (Case 4) and MSSA from abscess drainage (Case 5).
The dosage of dalbavancin for children is determined by age and should not exceed 1500 mg [14].
In this case series, the dose of dalbavancin administered was 18 mg/kg in infants aged 15 and 3 months (cases 3 and 5), while three patients older than 10 years (cases 1, 2 and 4) were given a dose of 22.5 mg/kg. All the patients received a single dose of dalbavancin.
A clinically relevant associated or potentially predisposing condition was reported in Case 1 (Clericuzio-type Poikiloderma with neutropenia syndrome). In this case, dalbavancin facilitated early discharge and resulted in significant improvement of skin lesions, consistent with the diagnosis of cellulitis, highlighting its efficacy in complex cases with underlying conditions predisposing to infectious complications.
Similarly, the Case 2 highlights the usefulness of dalbavancin in optimizing antibiotic therapy and facilitating outpatient management. The patient with bilateral periorbital cellulitis with pansinusitis caused by S. pneumoniae showed partial clinical improvement with conventional antibiotic therapy but required early discharge due to family needs. A single dose of dalbavancin ensured continuous and effective antibiotic coverage in the outpatient setting, leading to significant clinical improvement with a marked reduction of periorbital edema at follow-up.
Case 3 and Case 5 presented infants with cellulitis and abscess formation after vaccination. In both cases, administration of dalbavancin facilitated discharge, with significant clinical improvement, resolution of edema and reduction in abscess thickness.
In Case 4, dalbavancin was used in a patient with severe necrotizing pneumonia and extensive femoral abscess collections. Despite initial difficulties in managing the complex infection, dalbavancin played a crucial role in providing continuous and effective antibiotic coverage, leading to reduced abscess collections and improved bone condition.
The literature currently provides limited evidence regarding the use of dalbavancin for the treatment of ABSSSI in pediatric patients. Moreover, this drug does not yet appear to be widely used in clinical practice, as evidenced by the paucity of studies or case reports focusing on this topic. One exception is an Italian multicenter retrospective analysis focusing on the off-label application of dalbavancin in the treatment of bone infections and its in-label use for ABSSSI in children and adolescents [16]. However, it is important to note that the EMA, in approving dalbavancin for pediatric ABSSSI, relied on a robust literature in the adult setting and partial but very promising results from pediatric studies [17].
The challenge posed by multidrug-resistant (MDR) pathogens is expected to intensify in the coming decades, and children are likely to persist as a vulnerable population prone to acquiring infections caused by difficult-to-treat organisms, even in community settings [18].
Dalbavancin offers several advantages: high efficacy, excellent safety profile, possibility of early hospital discharge, prolonged and potent bactericidal activity against Gram-positive bacteria (including MRSA), spectrum of activity focused on a specific syndrome, and minimal need for monitoring due to its low drug-drug interaction potential [19].
The most attractive feature of dalbavancin treatment in these clinical cases is that it can be administered in the hospital immediately before discharge, allowing early discharge of the patient and providing an effective and time-saving alternative. This is clearly demonstrated in this study by comparing prolonged hospital stays before dalbavancin administration (length of stay ranged from 7 to 56 days, median 10 days) and rapid discharge after dalbavancin administration, which occurred within 1 day or on the same day.
Dalbavancin, as in adults, may be a viable option for selected pediatric patients with ABSSSI who are not suitable for oral antibiotics or outpatient parenteral antimicrobial therapy (OPAT) programs.
This is crucial considering the challenges associated with available oral therapies, such as multiple daily dosing affecting children’s adherence to therapy, potential drug interactions or side effects, and the need for laboratory monitoring. In addition, logistical constraints and the need for daily intravenous line use and therapeutic drug monitoring may limit the feasibility of OPAT strategies [20].
Another potential benefit of the use of dalbavancin in pediatric patients is improved quality of life, particularly for individuals with frequent healthcare system interactions, who would prefer to avoid hospitalization just for intravenous antibiotic administration, as shown in some cases of this study.
In addition, the use of this long-acting antibiotic can reduce the inconvenience associated with prolonged or repeated intravenous therapy by eliminating the need for daily venipunctures and the possible implantation of central venous catheters. This approach helps prevent complications associated with vascular access and reduces the likelihood of further nosocomial infections, as in Case 3, who contracted an Adenovirus infection during hospitalization.
Moreover, the use of dalbavancin is associated with significant cost savings, including reduced bed occupancy, highlighting its potential to mitigate the social and financial burdens of prolonged hospital stays in pediatric patients, as observed in a recent study that evaluated its impact on hospital length-of-stay and treatment-related costs in several Gram-positive bacterial infections [21, 22].
Due to its long-acting nature, potent antimicrobial action, and minimal need for monitoring, dalbavancin has proven to be a promising therapeutic option in pediatric patients with ABSSSI.
This is particularly notable in cases requiring prolonged antibiotic treatment or when adherence to alternative therapies poses challenges. In addition, the use of dalbavancin for the treatment of ABSSSI in pediatric settings may facilitate discharge from the hospital or even avoid unnecessary hospitalizations by being administered on an outpatient basis or in the Emergency Department, allowing a reduction in the period of hospitalization and related costs.
Although dalbavancin is currently approved only for the treatment of ABSSSI, the above considerations can be applied to several other infections caused by Gram-positive bacteria. These infections often require prolonged antibiotic therapy, so a long-acting drug could address a significant clinical need and improve compliance. Evidence of off-label use of dalbavancin in infections caused by S. aureus, such as arthritis, osteomyelitis, or endocarditis has reported favorable outcomes in the adult population [23]. Future clinical research should consolidate the safety and efficacy data of dalbavancin and extend the indications for use to other diseases, also in the pediatric setting, taking advantage of the drug’s pharmacokinetic peculiarities.
CONCLUSIONS
ABSSSIs are a frequent and serious condition among children, resulting in a high number of hospitalizations, increased risks of local and systemic complications, frequent need for surgical interventions, and prolonged exposure to broad-spectrum antibiotics. The prevalence of ABSSSI caused by multidrug-resistant pathogens, particularly MRSA, has increased significantly in recent decades, posing a substantial burden on pediatric patients. Currently, almost all treatment options require at least one hospitalization, multiple daily doses, and close monitoring due to safety concerns and potential drug interactions.
Dalbavancin is a long-acting agent with anti-MRSA activity recently approved for pediatric use. Although the evidence in this context remains rather limited, it suggests a favorable safety profile of the drug associated with strong efficacy and easy maneuverability. Consequently, dalbavancin might offer particular advantages for children with ABSSSI who are clinically stable (allowing early discharge) and have medium to high risk factors, such as recent hospitalization or underlying chronic diseases that predispose them to multidrug-resistant Gram-positive infections.
Conflict of interest
The authors declare no conflict of interest.
Funding sources
This study did not receive any funding from public or private agencies.
Acknowledgments
Medical writing assistance was provided by Dr. Isabella Esposito.
Informed consent
Written informed consent to publication of the details of these cases and the accompanying images were obtained from the patients involved in the study.
REFERENCES
[1] Esposito S, Bassetti M, Concia E, et al. Diagnosis and management of skin and soft-tissue infections (SSTI). A literature review and consensus statement: an update. J Chemother. 2017; 29(4): 197-214.
[2] Russo A, Concia E, Cristini F, et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin Microbiol Infect. 2016; 22 (Suppl. 2): S27-36.
[3] Leuthner KD, Buechler KA, Kogan D, Saguros A, Lee HS. Clinical efficacy of dalbavancin for the treatment of acute bacterial skin and skin structure infections (ABSSSI). Ther Clin Risk Manag. 2016; 12: 931-940.
[4] US Food and Drug Administration Acute bacterial skin and skin structure infections: developing drugs for treatment. Guidance for industry. 2013. Available at: https://www.fda.gov/files/drugs/published/Acute-Bacterial-Skin-and-Skin-Structure-Infections---Developing-Drugs-for-Treatment.pdf [accessed 8 March 2024].
[5] Pollack CV Jr, Amin A, Ford WT Jr, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med 2015; 48(4): 508-519.
[6] Mistry RD. Skin and soft tissue infections. Pediatr Clin North Am. 2013; 60(5): 1063-1082.
[7] Lopez MA, Cruz AT, Kowalkowski MA, Raphael JL. Trends in resource utilization for hospitalized children with skin and soft tissue infections. Pediatrics. 2013; 131(3): e718-e725.
[8] Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections Arch Intern Med 2008; 168(14): 1585-1591.
[9] Lautz TB, Raval MV, Barsness KA. Increasing national burden of hospitalizations for skin and soft tissue infections in children. J Pediatr Surg. 2011; 46(10): 1935-1941.
[10] Hedrick J. Acute bacterial skin infections in pediatric medicine: current issues in presentation and treatment. Paediatr Drugs. 2003; 5(1): 35-46.
[11] Moore SJ, O’Leary ST, Caldwell B, et al. Clinical characteristics and antibiotic utilization in pediatric patients hospitalized with acute bacterial skin and skin structure infection Pediatr Infect Dis J 2014; 33(8): 825-828.
[12] Esposito S, Noviello S, Leone S. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Infez Med. 2015; 23(4): 313-317.
[13] Giorgobiani M, Burroughs MH, Antadze T, et al. The safety and efficacy of dalbavancin and active comparator in pediatric patients with acute bacterial skin and skin structure infections. Pediatr Infect Dis J. 2023; 42(3): 199-205.
[14] Carrothers TJ, Lagraauw HM, Lindbom L, Riccobene TA. Population pharmacokinetic and pharmacokinetic/pharmacodynamic target attainment analyses for dalbavancin in pediatric patients. Pediatr Infect Dis J. 2023; 42(2): 99-105.
[15] Xydalba. EPAR - Product Information Available at: https://www.ema.europa.eu/en/documents/product-information/xydalba-epar-product-information_en.pdf [accessed 2 March 2024].
[16] Caselli D, Mariani M, Colomba C, et al. Real-World Use of Dalbavancin for treatment of soft tissue and bone infection in children: safe, effective and hospital-time sparing. Children (Basel). 2024; 11(1): 78.
[17] Gonzalez D, Bradley JS, Blumer J, et al. Dalbavancin pharmacokinetics and safety in children 3 months to 11 years of age. Pediatr Infect Dis J. 2017; 36(7): 645-653.
[18] Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019; 17(4): 203-218.
[19] Micheli G, Chiuchiarelli M, Taccari F, Fantoni M. The role of long-acting antibiotics in the clinical practice: a narrative review. Infez Med. 2023; 31(4): 449-465.
[20] Volpicelli L, Venditti M, Oliva A. Acute bacterial skin and skin structure infections in pediatric patients: potential role of dalbavancin. Expert Rev Anti Infect Ther. 2023; 21(4): 329-341.
[21] Poliseno M, Bavaro DF, Brindicci G, et al. Dalbavancin efficacy and impact on hospital length-of-stay and treatment costs in different Gram-positive bacterial infections. Clin. Drug Investig. 2021; 41(5): 437-448.
[22] Palmieri F, Alberici F, Deales A, et al. Early discharge of infectious disease patients: an opportunity or extra cost for the Italian Healthcare System? Infez Med. 2013; 21(4): 270-278.
[23] Esposito S, Pagliano P, De Simone G, et al. In-label, off-label prescription, efficacy and tolerability of dalbavancin: report from a National Registry. Infection. 2024 doi: 10.1007/s15010-024-02176-2. Online ahead of print.