Le Infezioni in Medicina, n. 2, 222-230, 2024
doi: 10.53854/liim-3202-10
ORIGINAL ARTICLES
Mycoplasma genitalium prevalence, co-infection and macrolide resistance-associated mutations in Southern Vietnam
Pham Phuoc Hung Lam1, Ngoc Hieu Nguyen2, Thi Thanh Tho Nguyen1, Ngo Binh Trinh2, Bac An Luong2
1Ho Chi Minh City Hospital of Dermato-Venereology, Ho Chi Minh City, Vietnam;
2University of Medicine and Pharmacy at Ho Chi Minh city, Vietnam
Article received 7 February 2024, accepted 29 April 2024
Corresponding author
Ngoc Hieu Nguyen
E-mail: ngochieu0707@gmail.com
SummaRY
Mycoplasma genitalium is an emerging sexually transmitted infection, with increasing rates of macrolide resistance and some ways of treatments being recommended by many countries. This study aimed to investigate the prevalence of M. genitalium infection, M. genitalium co-infection with other sexually transmitted organisms, and the frequency of macrolide antibiotic resistance genotypes identified in urethral specimens collected from male and urethral, vaginal and cervical specimens from female who visited the STIs clinic of HCMC Hospital of Dermato-Venereology, Vietnam. The results obtained positive samples for C. trachomatis was 8.46%, N. gonorrhoeae was 6.28%, and M. genitalium was 5.95%. Fifty-five out of 90 M. genitalium samples were found to have mutations in the 23S rRNA gene associated with macrolide resistance (61.11%). M. genitalium/C. trachomatis co-infection was 6.19%, and M. genitalium/N. gonorrhoeae was 1.22%. The percentage of M. genitalium carrying the macrolide resistance mutant gene co-infected with C. trachomatis accounted for 37.50%. The high prevalence of the M. genitalium mutations associated with macrolide resistance showed the importance of M. genitalium testing.
Keywords: Mycoplasma genitalium, Chlamydia trachomatis, Neisseria gonorrhoeae, co-infection, macrolide resistance.
INTRODUCTION
According to data from the World Health Organization (WHO), more than one million patients suffer from sexually transmitted infections (STIs) a day. Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are the most common sexually transmitted infections globally [1, 2]. In recent years, Mycoplasma genitalium (MG) infection has gained much attention with similar symptoms of CT and NG infection [3]. MG is a small, slow-growing intracellular bacterium with no cell wall and the smallest genome (580kb) for a self-replicating organism. MG transmission occurs by genital-genital or penile-anal contact, less likely with oral-genital contact [3-5]. The prevalence of MG infection in the global population is estimated to be 1-4% in men and 1-6.4% in women, being even more common in STI testing centers (4-38%) [6]. These studies showed that the prevalence of MG infection is approximately 1% in the screening population, and ranges from 9% to >50% in populations at high risk for STIs [7-10].
Traditional treatment of MG has been a single dose of 1 g azithromycin, and using moxifloxacin is recommended as a second-line agent. However, the recent increase in the number of MG cases as well as the significant increase in the resistance to both drugs [11, 12] leads to treatment failure [11-13]. MG is intrinsically resistant to beta-lactams and other antibiotics acting on the cell wall, limiting alternative treatment options. Because MG is very difficult to culture, monitoring for genetic markers of antibiotic resistance has become a key tool of surveillance in clinical monitoring of susceptibility to MG. The MG resistance to macrolides is associated with mutations in the 23S rRNA gene. The prevalence of the macrolide-resistant MG has recently increased significantly around the world [14] and varies across geographic regions, for example, 18% in Stockholm, Sweden [15], 41% in London, UK [16], 41.4% in Copenhagen, Denmark [17], 47.3% in Canada [18], 52.6% in Dresden, Germany [19].
In addition, the data of co-infection of MG with other sexually transmitted bacteria is limited. According to the 2016 US study, the rate of co-infection of MG with CT was 22.13%, with NG was 15.38% and with both of them was 0.68% [20]. When the co-infection occurs, treatment with a single dose of azithromycin 1g increases the risk of mutations in the macrolide resistance gene of MG, making it difficult to treat MG [16]. Therefore, during the treatment of NG and CT, it is necessary to consider the presence of MG to provide a suitable treatment regimen for patients.
In Vietnam, there are still not many studies describing the clinical characteristics, relationships, and pathogenic role of MG, which makes it difficult to treat. The aim of the study was to investigate the proportion of patients infected with MG and its macrolide resistance-associated mutations, and the co-infection of MG with CT or NG at the STIs clinic, HCMC Hospital of Dermato-Venereology, Vietnam.
PATIENTS AND METHODS
Study population
All samples were taken from patients (≥18 years old) who visited the STIs clinic of HCMC Hospital of Dermato-Venereology, Ho Chi Minh City, vIETNAM and were approved by the hospital’s medical ethics committee. Specimens were obtained from symptomatic patients (vaginitis, cervicitis, dysuria, pelvic inflammatory disease in women and urethral discharge, itching, painful urination in men) and from patients without symptoms (including patients who came to the examination for other conditions such as syphilis, genital warts, genital fungal infections... or had a sex partner who got a sexually transmitted disease or only went to the hospital for screening) visited the STIs clinic from February to November 2022. The demographics and symptom status of each patient were determined by the physician at the time of patients visit to the hospital clinic. In addition, we investigated whether the patients took azithromycin during their illness (the duration of illness was conventional from the onset of the first symptom to the time of the current examination).
Samples were taken from the urethral, vaginal, and cervical swabs with cotton swabs. The swab sample were transferred into an Eppendorf tube containing 0.5mL of sterile 0.9% NaCl solution or 0.3mL of transport medium. 200μL of the sample were put into 10μL internal control, then the mixture was put into sample tube, the sample transferred to SaMag-12 machine tray (Sacace Biotechnologies) to obtain total DNA. Then to identify CT, NG and MG by real-time PCR method (Mx3005P-Agilent Technologies), samples were mixed with Multiplex kit (Sacace). The results were read by using linear scale analysis.
Sequencing
Macrolide resistance of MG was detected by using reverse transcription-PCR of the 23S rRNA region of MG, which was performed as previously described and followed by sequencing Sanger [21]. This gene was amplified by using 23S-F (5’ CCATCTCTTGACTGTCTCGGCTAT 3’) và 23S-R (5’ AATCCTTGCGAACTTGCATC 3’) and HotStar Taq (12.5μL HotStar Taq Buffer (Qiagen), 1μM primer, 5μL extracted DNA, in 30μL reaction). The gene sequencing primer used was PCR amplification primer and obtained a sequence of 150nt. Centrifugation conditions were 95°C for 15min, then 45rpm at 94°C for 15s, 58°C for 30s, 72°C for 30s, and finally 3min at 72°C. To detect mutations at positions 2058 and 2059 (Escherichia coli numbering; located in region V of the 23S rRNA) that were associated with MG macrolide resistance [22], we introduce the sequence automatically awarded to GenBank.
Statistical analyses
For all sexually transmitted organisms, subjects were considered infected if their specimen was positive for one or more microorganisms. The infection rate was calculated using the infection status criterion. All tests for 95% confidence intervals (CIs) were two-sided and performed at the 0.05 significance level, using the efficient score method. Odds ratio (OR) calculation and significance test were performed as described previously [23], with P value <0.05 considered significant.
RESULTS
Prevalence of M. genitalium với C. trachomatis and N. gonorrhoeae infections
The proportion of patients infected with MG, CT, and NG in 1513 patients who visited the STIs clinic was classified by sex, and age group (Table 1). The prevalence of CT, NG, and MG infection was 8.46%, 6.28%, and 5.95%, respectively.
Table 1 - Prevalence of M. genitalium, C. trachomatis and N. gonorrhoeae by subject age group, self-identified demographic status.
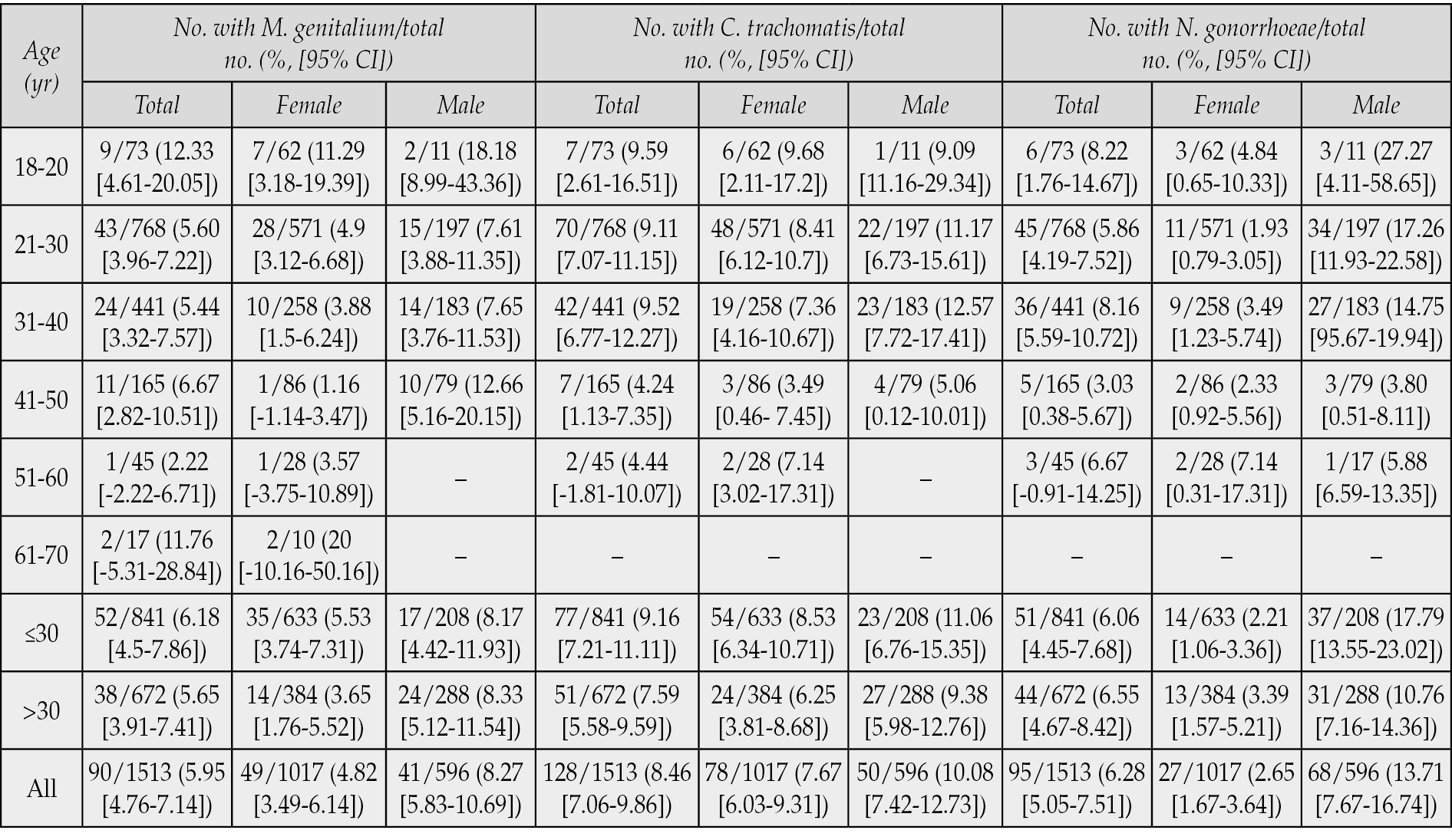
The prevalence of MG infection was 5.95% [95% CI, 4.76 to 7.14] and the prevalence was higher in men than in women (8.27% in male vs 4.82% in female [OR,1.78; P=0.01]). The patients aged ≤30 years and aged >30 years MG-infected were 6.18% [95% CI, 4.5 to 7.86] and 5.65% [95% CI, 3.91 to 7.41], respectively. There was no difference in the prevalence in both men and women in these two age groups (female: 5.53% vs 3.65% [OR, 1.5; P=0.2265], male: 8.17% vs 8.33% [OR, 0.98; P=1,000]). The prevalence of MG infection in females was highest between the ages of 18-20 years (11.29%, [95% CI, 3.18 to 19.39]). In males, the prevalence of MG was high in two age groups 18-20 years old (18.18% [95% CI, 8.99 to 43.36] and 41-50 years old (12.66% [95% CI, 5.16 to 20.15]).
The prevalence of CT infection was 8.46% [95% CI, 7.06 to 9.86], in which this prevalence in females was 7.67% [95% CI, 6.03 to 9.31], and in males was 10.08%. 95% CI, 7.42 to 12.73]. The patients with CT infection at age ≤30 and >30 were statistically non-significant difference (total: 9.16% vs 7.59% [OR, 1.23; P=0.3067]; female: 8.53% vs 6.25% [OR, 1.39; P=0.2241], male: 11.06% vs 9.38% [OR, 1.201; P=0.5489]). The rate of CT infection was the highest in women aged 18-30 years (9.68% in 18-20 age and 8.41% in 21-30 age) and in men from 21-40 years old (11.17% in 21-30 years old and 12.57% in 31-40 years old).
Meanwhile, the prevalence of GN infection was 6.28% [95% CI, 5.05 to 7.51], in which this prevalence in females was 2.65% [95% CI, 1.67 to 3.64], and in males was 13.71% [95% CI, 7.67 to 16.74]. There was a statistically non-significant difference in GN patients aged ≤30 years and >30 years old (total: 6.06% vs 6.55% [OR, 1.227; P=0.3067]; female: 2.21% vs 3.39% [OR, 1.39; P=0.2241]), male: 17.79% vs 10.76% [OR, 1.202; P=0.5489]). The high prevalence of GN infection in males was in the ages of 18-40.
The results from Figure 1 showed that the proportion of patients infected with MG and GN in the symptomatic group was higher than in the asymptomatic group (MG: 6.87% vs 3.87% [OR, 1.83; P=0.0248) and GN: 7.82% vs 2.80% [OR, 2.95; P=0.0001]), while the prevalence of CT infection in these two groups was the statistically non-significant difference (9.26% vs 6.67% [OR,1.43; P=0.1089]). In males, the proportion of patients with these bacteria was higher in symptomatic subjects than in asymptomatic ones (MG: 10.43% vs 1.64% [OR, 6.985; P=0.0010]; CT: 12.30 % vs 3.28% [OR, 4.14; P=0.0029] and GN: 17.38% vs 2.46% [OR, 8.34; P<0.0001]). In women, there was no difference in the prevalence of MG, CT, and GN infection in these two groups.
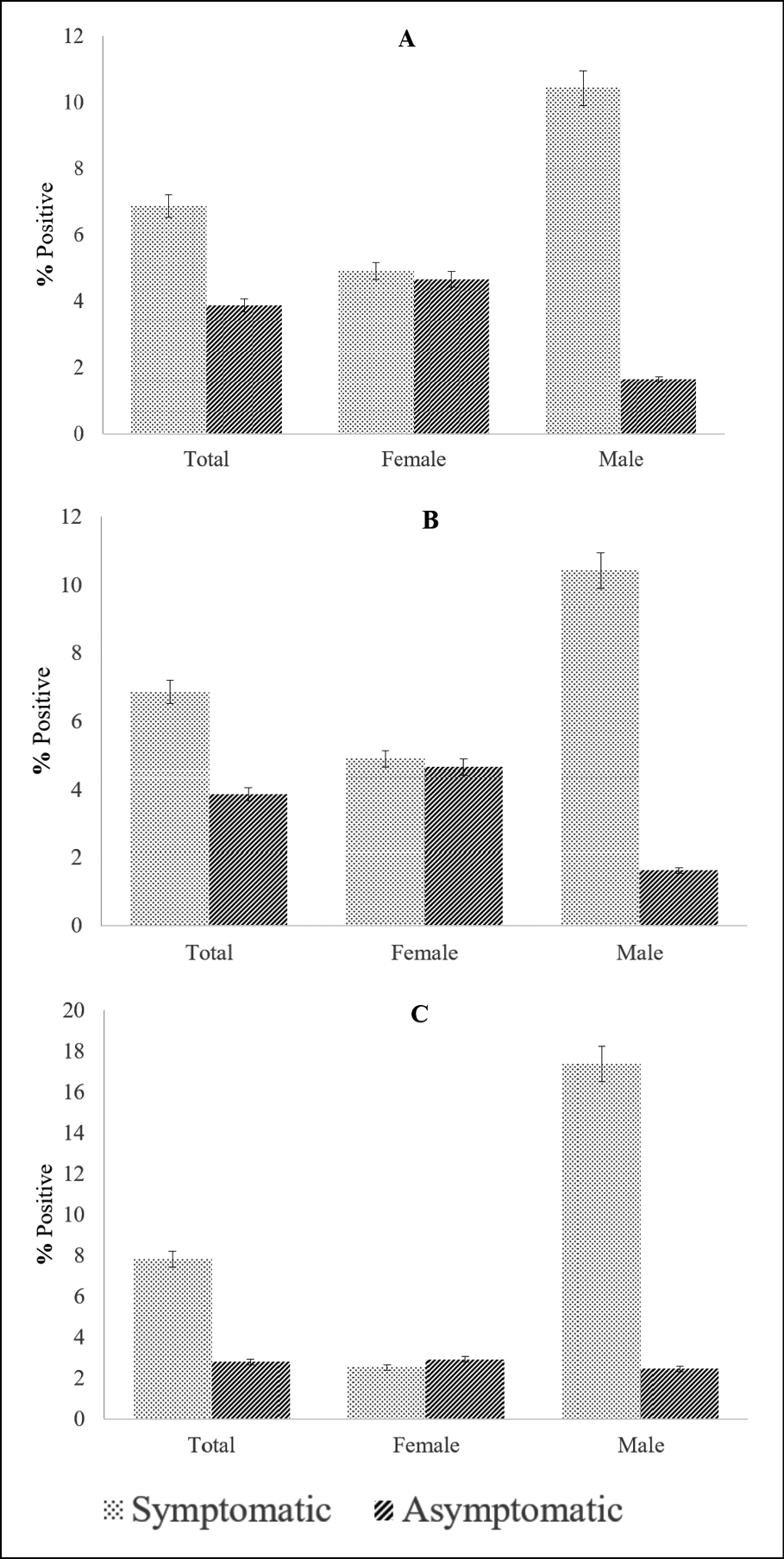
Figure 1 - The prevalence of patients tested for M. genitalium (Fig. A), C. trachomatis (Fig. B) and N. gonorrhoeae (Fig. C) in symptomatic and asymptomatic groups.
Rates of co-infections of M. genitalium with C. trachomatis and N. gonorrhoeae
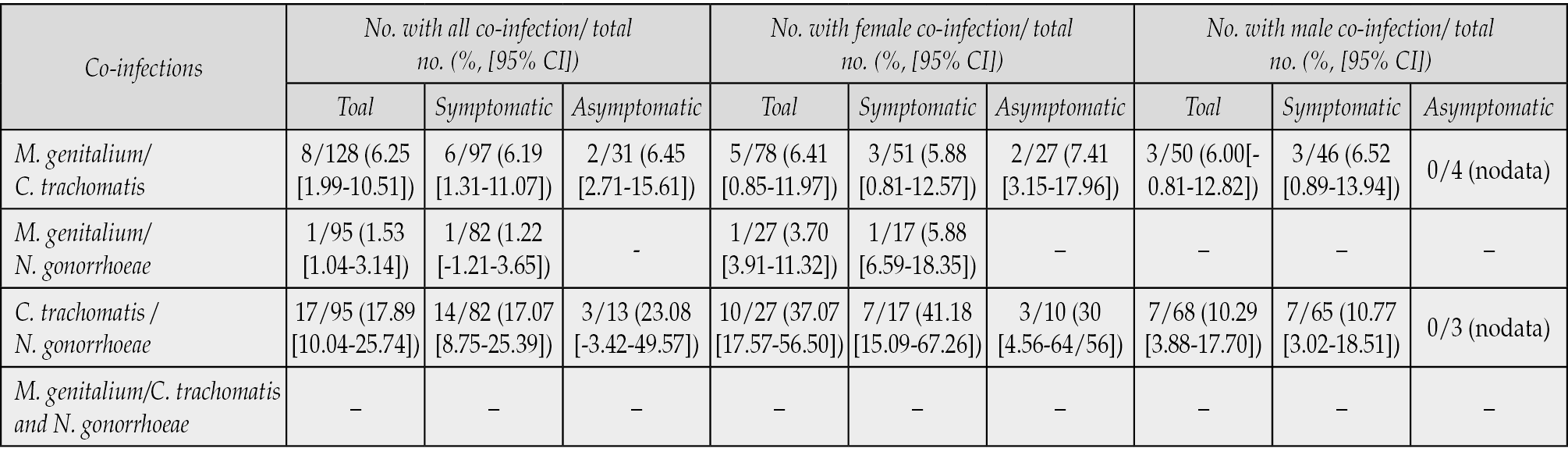
In 1513 patients who visited the STIs clinic, there were 90 patients with MG, 128 patients with CT, and 95 patients with NG (Table 2). The proportion of patients coinfected with MG/CT was 6.25% [95% CI, 1.99 to 10.51], MG/GN was 1.53% [95% CI, -1.04 to 3.14], CT/NG was 17.89% [95% CI,10.04 to 25.74] and no patient co-infected with all three bacteria. The prevalence of MG/CT co-infection in men and women respectively was 6.00% [95% CI, -0.81 to 12.82] and 6.41% [95% CI, 0.85 to 11.97]. The rate of CT/NG co-infection was higher in women than in men (female: 37.07% and male: 10.29%). Meanwhile, MG/GN co-infection in this study was only detected in a female case. MG/CT co-infection in symptomatic and asymptomatic patients was respectively 6.19% [95% CI, 1.31 to 11.07] and 6.45% [95% CI, -2.71 to 15.61]. MG/GN co-infection was detected in only one case in symptomatic patients. The rates of CT/MG co-infection in symptomatic and asymptomatic patients were 17.07% [95% CI, 8.75 to 25.39] and 23.08% [95% CI, -3.42 to 49.57] respectively.
Table 2 - Prevalence co-infection of M. genitalium in C. trachomatis and N. gonorrhoeae positive samples.
Rates of macrolide antibiotic resistance markers
Reverse transcription-PCR and Sanger sequencing at the 23S rRNA fragment of MG were used to find macrolide resistance mutations in 90 MG-positive samples (Table 3). The proportion of patients infected with MG-carrying mutations was 61.11% [95% CI, 50.84 to 71.38], these mutations occurred at two positions 2058 and 2059 in 23S rRNA (A2058G: 16.36% [6.27 to 26.45], A2058T: 21.81% [10.55 to 33.08] and A2059G: 65.45% [52.48 to 78.43]). The MG-infected women carried the same mutation as in the males (61.22% vs 60.98% [OR, 1.01; P=1,000]). The mutation at position A2059G was the most common in both females and males infected with MG-carrying mutations (female: 66.67% [95% CI, 49.76 to 84.57]; male: 64.00% [95% CI, 43.78 to 84.2]).
Table 3 - M. genitalium macrolide antibiotic resistance marker frequency in patients subjects.
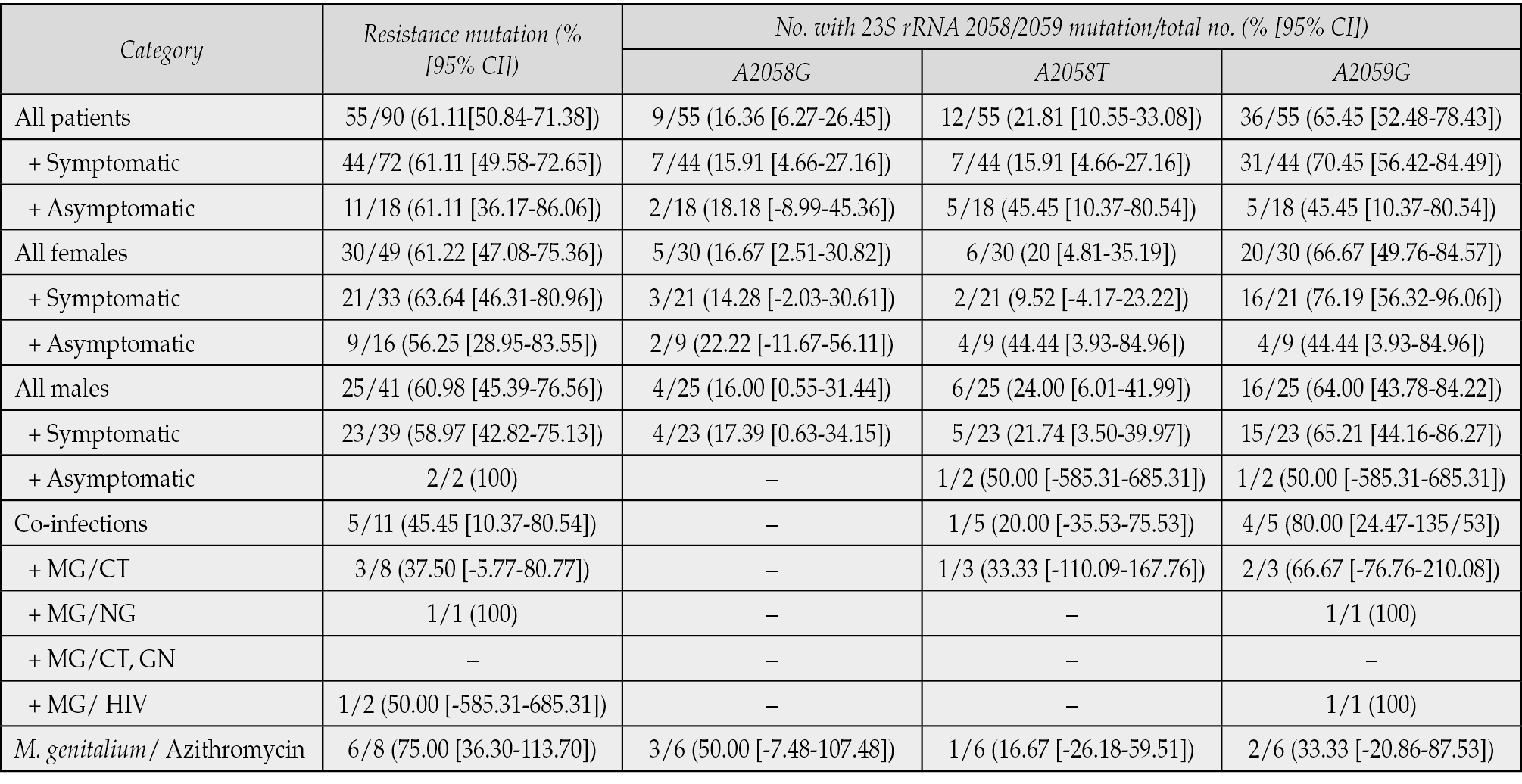
In symptomatic and asymptomatic patients, the rates of infection with MG carrying a mutated gene were similar (61.11%). In male, the rate in the symptomatic group was 58.97% [95% CI, 42.82 to 75.13] and was 100% in the other (2/2 cases). In females, the rate in the symptomatic group was 63.64% [95% CI, 46.31 to 80.96], and in the asymptomatic group was 56.25% [95% CI, 28.95 to 83.55]. In symptomatic patients with MG, the point mutation at the A2059G site was highest in both sexes (female: 76.19% [95% CI, 56.32 to 96.06] and male 65.21% [95% CI, 44.16 to 86.27)]). In the group of asymptomatic patients, the A2058T and A2059G point mutations were similar (female: 44.44% [95% CI, 3.93 to 84.96] and male: 50.00% [95% CI, -585.31to 685.31]). In this study, mutations at both A2058T and A2059G were seen in only two patients (1 with symptoms and 1 without symptoms).
The percentage of MG carrying the macrolide resistance mutant gene in MG/CT, MG/GN and MG/HIV coinfected patients was respectively 37.50% (3/8), 100% (1/1), and 50% (1/2). In particular, for the patients who used azithromycin during MG infection (before the time of taking samples for testing), the rate of MG infection carrying mutant genes accounted for 75.00% [95% CI, 36.30 to 113.70] (6/8), in which the point mutation A2058G was 50%, A2058T was 16.67%, and A2059G was 33.33%.
DISCUSSION
In this study, we evaluated the prevalence of MG, CT, and GN infection among subjects who visited the STIs clinic of HCMC Hospital of Dermato-Venereology, Ho Chi Minh City, Vietnam, and this was the data representing the largest geographic population in the region of southern, Vietnam. The results showed that the proportion of men infected with MG, CT, and GN was higher than that of women. This may be because men tend to have sex with more partners than women.
In this study, the infection rate of these three microorganisms did not differ between the two age groups ≤30 and >30 years old. Meanwhile, many previous reports showed that the prevalence of STIs was high among young people and then declined with increasing age such as in the United States, the European Community, Asia, Oceania and Africa [8, 9, 24-33]. Therefore, the trend of unsafe sex such as multiple partners, not using condoms in Vietnam might occur at all ages in the survey. In addition, this study also showed that the rate of infection with the three bacteria was higher in symptomatic patients than in the asymptomatic group, and most of the infected men was symptomatic. However, half of the infected women were symptomatic, the rest progressed silently without any symptoms. To our knowledge, in Vietnam, very few people including sex workers without symptoms wanted to undergo periodic screening.
In the world, there have been many studies on the rate of MG co-infection with CT and NG [20, 34, 35]. However, in Vietnam, there was only one retrospective report on the co-infection rate of MG/CT [36]. Our results showed that the rate of co-infection with MG/CT (6.25%), MG/NG (1.53%), CT/NG (17.89%) and no cases of co-infection with all three bacteria. In Southeast Asia, Singapore reported a prevalence of MG/CT co-infection (8.1%), and no cases of MG/NG co-infection or co-infection of all three bacteria were detected [34]. In our study, 95 cases of NG infection were recorded, but only 1 woman had co-infection with MG. The cause may be due to the narrow urethra in men, strong growth of NG (manifested in aggressive symptoms), which made a biological competition between MG and NG. The result was that MG will not be able to survive in a microenvironment with the existence of NG.
In MG infection, azithromycin has been widely used for the treatment [37]. In recent years, many studies have reported an increasing incidence of MG macrolide resistance gene mutations [16, 22, 38]. In this study, we also found that the rate of MG carrying the macrolide resistance mutant gene accounted for 61.11% (61.22% in women and 60.98% in men) and higher than many other studies (Singapore (25%), Denmark (56%), USA (48.3%) [20, 34, 35]. A study in Australia found that 56% of MG infections originating in Southeast Asia failed treatment with azithromycin [39]. The investigation of MG carrying the macrolide resistance mutant gene in Vietnam was firstly conducted by our study, and we believe that this is an important data to assist clinicians in selecting appropriate antibiotics. This resistance is related to genetic mutations in the 23S rRNA region at two positions 2058/2059 [40], mutations at both sites lead to very high levels of azithromycin resistance [40]. Our study showed that A2059G (65.45%) mutation was more dominant than A2058T (21.81%) and A2058G (16.36%). Unemo’s study also showed that the 23S rRNA gene mutation at the A2059G position was dominant (53.5% in Denmark and 59.6% in Norway) [17].
The first-line treatment of CT is oral azithromycin 1g which has been shown to induce MG macrolide resistance in co-infected subjects [3]. Therefore, the importance of evaluating MG and CT co-infection was emphasized in this study, the rate of MG/CT co-infection was 6.25% and the rate of MG carrying the mutated gene in this co-infection was 37.50%. Reports in Denmark and Singapore showed that the point mutations in the 23S region related to macrolide resistance accounted for 64% and 55.56%, respectively [34, 35]. MG is rarely diagnosed in Vietnam and is therefore rarely treated specifically, whereas first-line CT therapy is single-dose azithromycin. Although single-dose macrolide therapy has previously been advocated for MG, there is growing evidence that a 5-day course of macrolides should be used to avoid resistance and ensure eradication of MG completely (removal of about 95%) [13, 41]. From that, it is necessary to consider the existence of MG before deciding to treat CT.
In addition, in two cases of MG/HIV co-infection, there was one case of MG infection carrying a mutant gene for macrolide resistance in our study. Belén et al. found that MG/HIV co-infected patients had 73.7% MG carrying macrolide resistance mutations [42]. According to Dionne-Odom et al.’s study, this rate was 74.1% in HIV-MSM patients in Alabama [43]. However, our sample size as well as the other studies was limited, it is necessary to expand the study on HIV subjects to have reliable data.
A recent study reported that selecting during treatment with azithromycin 1g resulted in 55% of MG treatment failures [44]. In this report, we showed that the rate of MG infection carrying the mutant gene for macrolide resistance in the patients who had taken azithromycin before was up to 75%. This high mutation rate indicated that MG easily creates the drug resistance mutations and after the administration of azithromycin [45]. This showed the importance of diagnostic testing for MG and when patients with MG have been previously treated with azithromycin, it should not be taken again. According to the 2021 European guideline, the treatment for macrolide-resistant MG infection is moxifloxacin. The persistent MG infection after azithromycin and moxifloxacin can be treated with doxycycline, minocycline (the cure rate of 40 to 70%) or pristinamycin (the cure rate of around 75%) [46].
Author contribution statement
Pham Phuoc Hung Lam and Ngoc Hieu Nguyen: conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Thi Thanh Tho Nguyen and Ngo Binh Trinh, Bac An Luong: Analyzed and interpreted the data, wrote the paper.
Acknowledgements
The authors would like to give thanks for the support of the HCMC Hospital of Dermato-Venereology and University of Medicine and Pharmacy at Ho Chi Minh City for creating conditions for the research team to carry out the research at the hospital.
Conflict of interest
No benefits have been or will be received from a commercial party related directly or indirectly to the subject matter of this article.
Funding
None to declare.
REFERENCES
[1] Chan PA, Robinette A, Montgomery M, et al. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol. 2016; 2016.
[2] McConaghy JR, Panchal B. Epididymitis: an overview. Am Fam Physician. 2016; 94(9): 723-726.
[3] Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016; 30(10): 1650-1656.
[4] García-Morales L, González-González L, Querol E, Piñol J. A minimized motile machinery for Mycoplasma genitalium. Mol Microbiol. 2016; 100(1): 125-138.
[5] Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011; 24(3): 498-514.
[6] Cazanave C, Manhart LE, Bébéar C. Mycoplasma genitalium, an emerging sexually transmitted pathogen. Med Mal Infect. 2012; 42(9): 381-392.
[7] Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007; 97(6): 1118-1125.
[8] Gatski M, Martin DH, Theall K, et al. Mycoplasma genitalium infection among HIV-positive women: prevalence, risk factors and association with vaginal shedding. Int J STD AIDS. 2011; 22(3): 155-159.
[9] Wetmore CM, Manhart LE, Lowens MS, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-comparison study. Sex Transm Dis. 2011; 38(3): 180.
[10] Lillis RA, Martin DH, Nsuami MJ. Mycoplasma genitalium infections in women attending a sexually transmitted disease clinic in New Orleans. Clin Infect Dis. 2019; 69(3): 459-465.
[11] Manhart LE, Jensen JS, Bradshaw CS, Golden MR, Martin DH. Efficacy of antimicrobial therapy for Mycoplasma genitalium infections. Clin Infect Dis. 2015; 61 (Suppl. 8): S802-S817.
[12] Murray GL, Bradshaw CS, Bissessor M, et al. Increasing macrolide and fluoroquinolone resistance in Mycoplasma genitalium. Emerg Infect Dis. 2017; 23(5): 809.
[13] Lau A, Bradshaw CS, Lewis D, et al. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis. 2015; 61(9): 1389-1399.
[14] Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol. 2017; 14(3): 139-152.
[15] Björnelius E, Magnusson C, Jensen JS. Mycoplasma genitalium macrolide resistance in Stockholm, Sweden. Sex Transm Infect. 2017; 93(3): 167-168.
[16] Pond MJ, Nori AV, Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014; 58(5): 631-637.
[17] Unemo M, Salado-Rasmussen K, Hansen M, et al. Clinical and analytical evaluation of the new Aptima Mycoplasma genitalium assay, with data on M. genitalium prevalence and antimicrobial resistance in M. genitalium in Denmark, Norway and Sweden in 2016. Clin Microbiol Infect. 2018; 24(5): 533-539.
[18] Chernesky MA, Jang D, Martin I, et al. Mycoplasma genitalium antibiotic resistance–mediating mutations in Canadian women with or without Chlamydia trachomatis infection. Sex Transm Dis. 2017; 44(7): 433-435.
[19] Dumke R, Thürmer A, Jacobs E. Emergence of Mycoplasma genitalium strains showing mutations associated with macrolide and fluoroquinolone resistance in the region Dresden, Germany. Diagn Microbiol Infect Dis. 2016; 86(2): 221-223.
[20] Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol. 2016; 54(9): 2278-2283.
[21] Jensen JS. Protocol for the detection of Mycoplasma genitalium by PCR from clinical specimens and subsequent detection of macrolide resistance-mediating mutations in region V of the 23S rRNA gene. Methods Mol Biol. 2012: 129-139.
[22] Couldwell DL, Lewis DA. Mycoplasma genitalium infection: current treatment options, therapeutic failure, and resistance-associated mutations. Infect Drug Resist. 2015: 147-161.
[23] Altman DG. Practical statistics for medical research: CRC press; 1990.
[24] Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium compared to chlamydia, gonorrhoea and trichomonas as an aetiological agent of urethritis in men attending STD clinics. Sex Transm Infect. 2009; 85(6): 438-440.
[25] Björnelius E, Lidbrink P, Jensen JS. Mycoplasma genitalium in non-gonococcal urethritis-a study in Swedish male STD patients. Int J STD AIDS. 2000; 11(5): 292-296.
[26] Jensen JS, BjÖrnelius E, Dohn B, Lidbrink P. Comparison of first void urine and urogenital swab specimens for detection of Mycoplasma genitalium and Chlamydia trachomatis by polymerase chain reaction in patients attending a sexually transmitted disease clinic. Sex Transm Dis. 2004: 499-507.
[27] Nakashima K, Shigehara K, Kawaguchi S, et al. Prevalence of human papillomavirus infection in the oropharynx and urine among sexually active men: a comparative study of infection by papillomavirus and other organisms, including Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma spp., and Ureaplasma spp. BMC Infect Dis. 2014; 14(1): 1-8.
[28] Xiang Z, Yin Y-P, Shi M-Q, et al. Risk factors for Mycoplasma genitalium infection among female sex workers: a cross-sectional study in two cities in southwest China. BMC Public Health. 2012; 12: 1-6.
[29] Bradshaw CS, Tabrizi SN, Read TRH, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006; 193(3): 336-345.
[30] Mezzini TM, Waddell RG, Douglas RJ, Sadlon TA. Mycoplasma genitalium: prevalence in men presenting with urethritis to a S outh A ustralian public sexual health clinic. Intern Med J. 2013; 43(5): 494-500.
[31] Oliphant J, Azariah S. Cervicitis: limited clinical utility for the detection of Mycoplasma genitalium in a cross-sectional study of women attending a New Zealand sexual health clinic. Sex Health. 2013; 10(3): 263-267.
[32] Vandepitte J, Weiss HA, Bukenya J, et al. Association between Mycoplasma genitalium infection and HIV acquisition among female sex workers in Uganda: evidence from a nested case-control study. Sex Transm Infect. 2014; 90(7): 545-549.
[33] Pepin J, Labbé A-C, Khonde N, et al. Mycoplasma genitalium: an organism commonly associated with cervicitis among west African sex workers. Sex Transm Infect. 2005; 81(1): 67-72.
[34] Hart T, Tang WY, Chio MTW, Barkham T. Mycoplasma genitalium in Singapore is associated with Chlamydia trachomatis infection and displays high macrolide and fluoroquinolone resistance rates. BMC Infect Dis. 2020; 20(1): 1-6.
[35] Desdorf R, Andersen NM, Chen M. Mycoplasma genitalium prevalence and macrolide resistance-associated mutations and coinfection with Chlamydia trachomatis in Southern Jutland, Denmark. Apmis. 2021; 129(12): 706-710.
[36] Hieu NN, Hung LPP, Ngoc NPN. Mycoplasma Genitalium and Chlamydia Trachomatis Prevalence, Co-Infection and Relevant Factors in An Epidemiology Study (2016-2019) In Ho Chi Minh, Vietnam. National Journal of Community Medicine. 2023; 14(01): 65-70.
[37] Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015; 64(RR-03): 1.
[38] Salado-Rasmussen K, Jensen JS. Mycoplasma genitalium testing pattern and macrolide resistance: a Danish nationwide retrospective survey. Clin Infect Dis. 2014; 59(1): 24-30.
[39] Bradshaw CS, Jensen JS, Tabrizi SN, et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006; 12(7): 1149.
[40] Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium–positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008; 47(12): 1546-1553.
[41] Horner P, Ingle SM, Garrett F, et al. Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis. Sex Transm Infect. 2018; 94(1): 14-20.
[42] Belén R, Chloé LR, Jordana-Lluch E, et al. Detection and Prevalence of Macrolide and Fluoroquinolone Resistance in Mycoplasma genitalium in Badalona, Spain. Antibiotics (Basel). 2022; 11(4): 485.
[43] Dionne-Odom J, Geisler WM, Aaron KJ, et al. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis. 2018; 66(5): 796-798.
[44] Napierala M, Munson E, Wenten D, et al. Detection of Mycoplasma genitalium from male primary urine specimens: an epidemiologic dichotomy with Trichomonas vaginalis. Diagn Microbiol Infect Dis. 2015; 82(3): 194-198.
[45] Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998; 62(4): 1094-1156.
[46] Jensen JS , Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022; 36(5): 641-650.