Le Infezioni in Medicina, n. 2, 119-130, 2024
doi: 10.53854/liim-3202-1
REVIEWS
COVID-19 vaccine-associated lymphadenopathy: a review
Valeria Ciliberti1, Elisabetta Maffei1, Valentina Giudice2, Giuseppe Ciancia1, Pio Zeppa1, Alessandro Caputo1
1UOC di Anatomia Patologica, Azienda Ospedaliera Universitaria San Giovanni di Dio e Ruggi d’Aragona, University of Salerno, Italy;
2Hematology and Transplant Center, Azienda Ospedaliera Universitaria San Giovanni di Dio e Ruggi d’Aragona, University of Salerno, Italy
Article received 22 February 2024, accepted 30 April 2024
Corresponding author
Pio Zeppa
E-mail: pzeppa@unisa.it
SummaRY
Following the introduction of RNA-based vaccines, COVID-19 vaccine-associated clinical lymphadenopathy (C19-LAP) has been reported as a side effect. Moreover, subclinical lymphadenopathy detected on imaging (SLDI) has also been observed, mainly as incidental findings while performing screening tests on oncological patients. In these cases, surgical lymphadenectomy, fine-needle aspiration cytology (FNAC) and core needle biopsy (CNB) have been used as a valuable diagnostic tool for SLDI and C19-LAP. In this review the clinical, histologic and cytologic features of SLDI and C19-LAP have been investigated. A search for studies that reported on C19-LAP and SLDI histopathology and cytopathology was performed on PubMed and Google Scholar, on 11 January 2023. Thirty-one reports on SLDI and C19-LAP were retrieved and included in a pooled analysis. In total, we included 54 patients with a median age of 47 years. In our research, surgical excision, CNB and/or FNAC of C19-LAP or SLDI enlarged lymph nodes have been performed in 54 cases. Of all cases, only two metastases were diagnosed and one case was diagnosed as reactive hyperplasia with atypical follicles. The remaining cases were reactive lymphadenopathy (28 cases), follicular hyperplasia (13 cases), Kikuchi-Fujimoto disease (6 cases), granulomatous lymphadenitis (2 cases), eosinophilic lymph node abscesses (1 case), Langherans cell histiocytosis (1 case), Rosai-Dorfman disease (1 case). SLDI and C19-LAP have represented a diagnostic dilemma, especially in oncologic patients. The role of different diagnostic tools for SLDI and C19-LAP has been discussed.
Keywords: COVID, vaccine, lymphadenopathy, histology, cytology.
INTRODUCTION
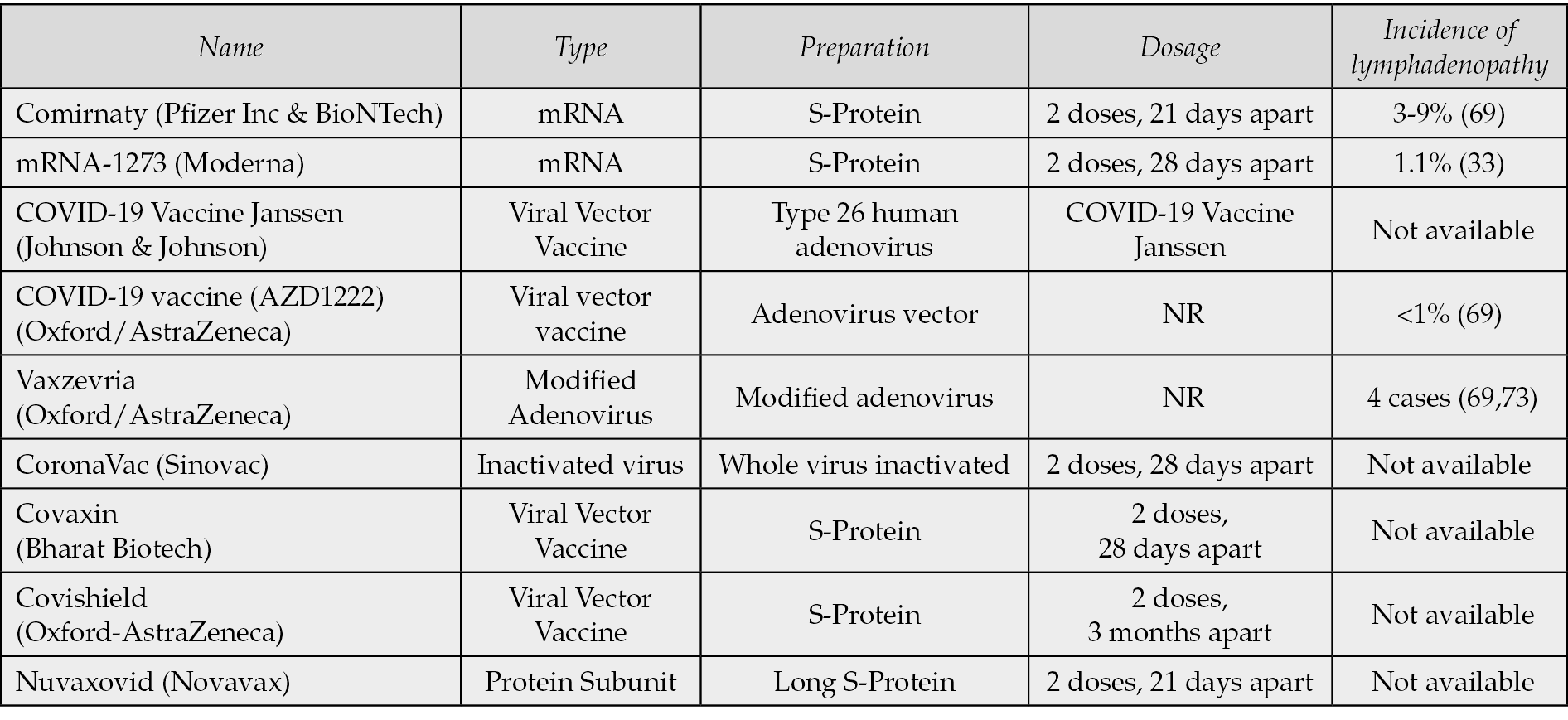
Whereas the COVID-19 has not been the first pandemic in human history, it has shown unique features in terms of diffusion and direct and indirect effects. Different vaccines have been produced and used in mass vaccination programs, with different modalities and different levels of efficacy on the populations for each country [1, 2]. Moreover, SARS-CoV-2 vaccines have been the first mRNA vaccines to be approved for clinical application. COVID-19 vaccines have been demonstrated to be safe and effective, with significant reduction in symptomatic COVID-19 in older adults, and with further protection against severe disease; however, some adverse effects have been reported [1-3]. Different COVID-19 vaccines have been developed. COVID-19 vaccines are commonly administered intramuscularly in the upper arm at determined intervals in at least two shots [2, 4]. As with any other vaccines, COVID-19 vaccine may cause side effects, the most common being local pain and inflammation at the injection site, fatigue, headaches, fever, chills, and muscle and joint pains, often registered after the first administration, also depending on the vaccine type and individual responsivity [2, 5-16]. Post-vaccinal lymphadenopathy due to reactive changes in the lymph nodes is well known and has been described as consequence of different vaccines including bacillus Calmette–Guerin (BCG), hepatitis B, human papillomavirus, and tetanus amongst several others [5-22]. COVID-19 vaccine-associated lymphadenopathy (C19-LAP) may also occur, mostly reported in axillary, clavicular or cervical lymph nodes, after vaccine inoculation in the arm. In the Pfizer BioNTech COVID-19 vaccine trial, the axillary and supraclavicular C19-LAP incidence occurring in the same side of injection was 0.3% for the vaccine group versus <0.1% for the placebo group [16, 22]. In the case of the Moderna vaccine trial, the incidence was 1.1% [16, 22]. The site of lymphadenopathy was axillary in 11% of the patients after the first dose, and 16% after the second dose of Moderna vaccine; similar data has been reported after the Comirnaty-Pfizer/BioNTech vaccine [23, 24]; Caputo et al. summarized the incidence of C19-LAP for each of COVID-19 vaccines (Table 1) [23-25]. C19-LAP is probably just the epiphenomenon of lymph node reactivity, since COVID-19 vaccine-associated subclinical lymphadenopathy (SLDI) has been reported in a higher percentage of cases when compared to C19-LAP [22]. The detection of C19-LAP is mainly clinical and can be confirmed by ultrasound (US). Combined clinical data and US features allow the diagnosis of reactive SLDI or C19-LAP; hence, just a clinical follow-up is appropriate in most cases. Nonetheless, some C19-LAP and SLDI mimicked malignant lymphadenopathies, raising differential diagnostic problems [26-32]. In these cases, a pathological evaluation of lymphadenopathy has been performed in a number of cases. A review of the pathological features of C19-LAP was performed by Chua et al. [14]. This review focused on the reported histopathological features of C19-LAP up to the 2021. Nonetheless, other than histopathology, core-needle biopsy (CNB) and fine-needle aspiration cytology (FNAC) may be used for both reactive processes and lymphoma or metastases and have been utilized to assess LAP during the pandemic [6, 25, 32-39]. In this study, a review of C19-LAP reports between January 2021 up to December 2023 has been performed including both cytological and histopathological reports which were analyzed according to the lymphadenopathy and vaccines types.
Table 1 - Main COVID-19 vaccines and corresponding lymphadenopathy as side effects, as reported by Caputo et al. [33]. The table was included with the permission of the authors.
MATERIALS AND METHODS
A literature search was initially performed through PubMed and Google Scholar, on 11 January 2024, with the following keywords: ‘COVID’, ‘vaccine’, ‘lymphadenopathy’, ‘histology’, ‘cytology’, and ‘fine-needle aspiration’. During the search, the authors placed no restrictions on the year of publication and searched reference lists of full-text articles, mainly those of systematic reviews for additional studies that were not identified in the initial search. Only literature published in English was selected, including studies that reported histopathological and/or cytological findings in COVID-19 vaccine-related lymphadenopathy [22, 25-34, 41-67]. Studies on SLDI, mainly detected by US or 18F-FDG PET-CT were also selected and used for a general comprehension and description of the phenomenon and to retrieve cases evaluated by histology or LN-FNAC [68-77]. Recommendation articles, protocols, commentaries, and non-English articles were not considered. Data extracted from studies regarding C19-LAP and SLDI included the following: type of publication, number of patients and clinical data, type and dose of administered vaccine, delay from last vaccination to lymphadenopathy, LN site and size, histological and cytological features, management and outcome.
COVID-19-vaccines lymphadenopathy
Whereas post-vaccinal LAP is a quite rare event, the majority of COVID-19 vaccines may cause reactive lymphadenopathy, which usually is subclinical and an occasional finding; nonetheless, in a minority of cases, C19-LAP may be clinically evident. In fact, 36% of cases presented increased lymph node 18F-FDG uptake up to 10 weeks after vaccination, with women and people over 65 years being most frequently affected [75, 76]. Lymph node enlargement has been reported in ~1% of the COVID-19 vaccinated, more specifically in 0.3% of Pfizer-BioNTech and 1.1% of Moderna vaccines, respectively (Table 1) [16, 41, 64]. While most SLDI do not show clinically evident lymph nodes enlargement, the awareness of physicians about SLDI is fundamental, especially in case of cancer staging or follow-up to avoid the risk of overdiagnosis.
Characteristics
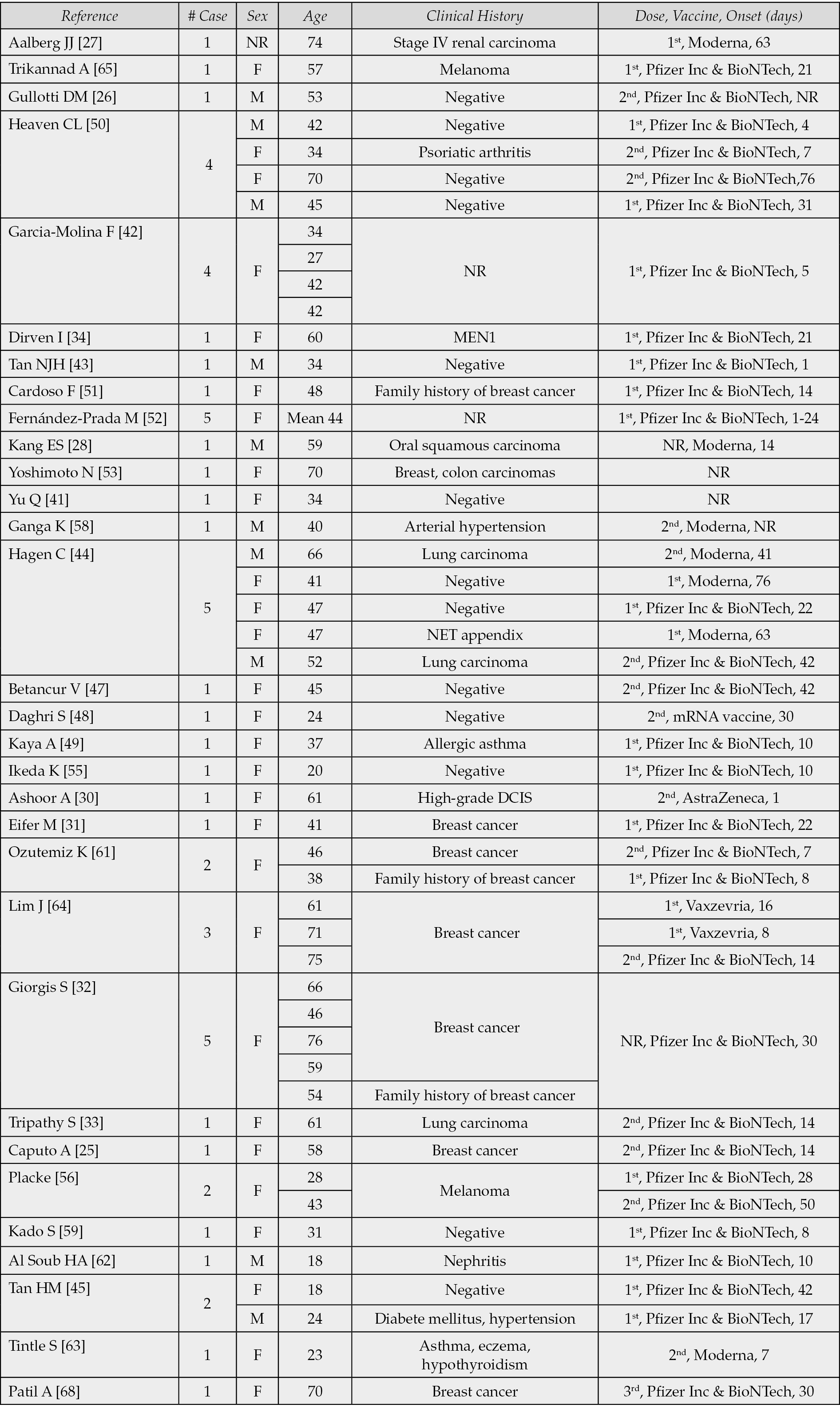
The present study is a pooled review based on 31 reports [25-28, 30-34, 41-45, 47-56, 58, 59, 61-65, 68]. In total, 54 cases are reported, including 43 (80%) females, 10 (18%) males, and one patient whose gender was not reported; the median age was 47 years. Previous or active history of different malignancies was reported in 24 cases (44%), which included breast cancer, renal cell carcinoma, melanoma, oral squamous cell carcinoma, neuroendocrine tumor (NET) or lung carcinoma or had a positive family history of breast carcinoma (2 cases, 4%) [21]. C19-LAP was reported after first, second or third administrations of the vaccine; Pfizer-BioNTech (40 cases, 74%) and Moderna (7 cases, 13%) were the most frequently used, followed by Vaxzevria (2 cases) and Astra-Zeneca (1 case), CureVac (1 case); in 1 case, vaccine type was reported as mRNA COVID-19 vaccine, while in 2 cases vaccine type was not reported. C19-LAP was observed in imaging examinations and after the first, second and third administrations of Pfizer-BioNTech vaccine; the time interval between the vaccine administration and the onset of the lymphadenopathy was reported in 35 out of 41 cases (85%), with median delay of 18.4 days (range, 1-76 days). As for Moderna, the median delay time was reported in 6 out of 7 cases (86%) and it was 44 days (range, 7-76). The median delay time for Vaxzevria was of 12 days, (range 8-16). Corresponding data are summarized in Table 2.
Histological and/or cytological examination was performed on 55 different lymph nodes. Reported lymphadenopathies were axillary (28 cases), supraclavicular (12 cases), cervical (6 cases), submandibular (3 cases), mediastinal (1 case), retro-auricular (1 case), inguinal (1 case) and scapular (1 case); lymphadenopathy site was not reported in 1 case (2%). All the lymph nodes were first evaluated by imaging, most frequently by ultrasound (US) and reported as enlarged, oval, usually hypoechoic, with major diameters ranging from 10 to 50 mm (mean 15.7); spherical shape was reported in 4 cases [44, 51, 63, 64]. Another reported US feature was diffuse or focal cortical thickening and preserved, visible hilum in almost all the cases. Increased standardized uptake value (SUV) was reported in 5 cases; effaced hilum was reported in one case [31, 33, 44, 56, 61, 68].
Table 2 - Clinical data of COVID-19 vaccines-related lymphadenopathies from 31 reports.
C19-lap pathological features
Surgical excision, CNB and/or FNAC of C19-LAP or SLDI have been performed in 54 cases [25-28, 30-34, 41-45, 47-56, 58, 59, 61-65, 68]. The pathological diagnoses were reactive lymphadenopathy (28 cases), follicular hyperplasia (13 cases), eosinophilic lymph node abscesses (1 cases), reactive hyperplasia with atypical follicles (1 case), granulomatous reaction (2 cases), metastases (2 cases), Kikuchi-Fujimoto disease (KFD) (6 cases), Langerhans cell histiocytosis (LCH) (1 case) [25, 27, 28, 30-32, 34, 41, 42, 44, 45, 47-52, 53, 56, 58, 59, 61-64, 65, 68]. In cases of reactive hyperplasia, the pathologist report described a preserved lymph node structure with cortical follicular hyperplasia, enlargement of germinal centers and interfollicular expansion by small lymphocytes. Prominent germinal centers and tingible-body macrophages were frequently reported; moreover, capillaries with focally prominent endothelial cells have been described in the expanded interfollicular regions [61]. Capsule thickening was frequently reported. The immunohistochemical phenotype (IHC) was reported in 12 cases [25, 26, 33, 42, 43, 45, 47, 48, 51-53, 55, 68]. Flow cytometry was reported in 7 cases [33, 42, 44, 51, 63]. Heaven et al. reported four cases of reactive lymphadenopathy: FNAC showed polymorphous lymphoid cells suggestive of a reactive process and the following histological examination confirmed the diagnoses [50]. Surgical excision was performed, assessing a polyclonal reactive process. Eifer et al. reported a case of axillary lymphadenopathy in a 41 years old woman with newly diagnosed breast cancer; hematoxylin- and eosin-stained images of cores of lymph node tissue showing prominently dilated and edematous sinuses that probably reflect reactive changes [30]. Ozutemiz et al. described a case of post-vaccine lymphadenopathy in a 46 years old woman with a history of breast cancer [61]. Histopathology was consistent with reactive lymph nodes. García-Molina F et al. reported 2 cases of nonspecific chronic adenitis [42]. Tan NJH et al. described one case of reactive follicular hyperplasia [43]. Fernández-Prada M et al. reported 5 cases showing reactive inflammatory signs, with lymphocytic infiltrate and active germinal centers [52]. Patil et al. described a case of atypical follicular hyperplasia with light chain–restricted germinal centers after COVID-19 Pfizer-BioNTech vaccine booster [68]. In histologic section, lymph node contained prominent and abnormal secondary lymphoid follicles, which showed ill-defined borders, poorly defined to absent mantle zone, lack of germinal polarization and contain a relatively monotonous population of medium- to large-sized centrocytic and centroblastic lymphoid cells with decreased apoptotic bodies and no tingible body macrophages. Kaya et al., instead, reported a case of C19-LAP with eosinophilic abscesses observed in a 37 years old female patient with a history of allergic asthma [49]. COVID-19 associated KFD have been described as typical histological features of corresponding, non-vaccinal related entities [45, 47, 48, 55, 62]. Core needle biopsies of lymph nodes showed multifocal necrotizing lymphadenopathy characterized by foci of necrosis surrounded by reactive appearing small lymphoid cells, histiocytes and plasma cells. Placke et al. and Trikannad et al. both reported a case of granulomatous reactive process in patients undergoing staging for melanoma [56, 65]. Tintle et al. reported the histological features of a case of post-vaccination Langerhans cell hyperplasia in a 23-years-old woman [63]. The authors reported focal aggregates of LCs, dendritic cells, and histiocytes with rare images of hemophagocytosis. Gullotti et al. and Tripathy et al. reported metastases for melanoma and lung cancer, respectively diagnosed by FNAC [26, 33].
In summary, pathological evaluation of SLDI and C19 LAP has been performed during the follow-up for different neoplasms in 24 patients, in 6 patients with comorbidity, in 15 cases with negative clinical history and in 9 cases in which patients’ clinical history was not reported. Clinical and pathological features of the reported cases are summarized in Table 3.
Table 3 - Imaging and pathological features of COVID-19 vaccine related lymphadenopathies from 31 reports.

DISCUSSION
Post-vaccine lymphadenopathy
Post-vaccine lymphadenopathy is a well-known phenomenon which may occur as a side effect of different vaccines, sometimes simulating a lymphoma, either clinically or pathologically. SARS-CoV-2 vaccines have been the first mRNA vaccines administered on large-scale [5-15, 35, 68, 73]. These vaccines base their mechanism of action on mRNA delivered into host cells, where it is translated into a protein then targeted by the immune system [2, 16, 83, 84]. The mRNA COVID-19 vaccine’s high immunogenicity might explain the higher rates of LAP, reported as side effects, when compared to other vaccines [36, 80, 81]. Assessing the real incidence of C19-LAP may be difficult, in particular for the heterogeneity of the sampled vaccinated populations, the lack of systematic investigations and the selection bias. Moreover, an additional factor impacting the evaluation of the incidence of SLDI and C19-LAP is caused by the presence of patients who undergo imaging evaluation for pre-existing morbidities and for staging or follow-up of neoplastic diseases by 18F-FDG PET-CT, in which SLDI are more likely to be detected [35, 36, 53, 61, 72, 81]. The nature of the vaccine may contribute to the morphological features of post-vaccine lymphadenopathies [11-15, 19, 67, 82, 83].
Pathology of COVID-19 post-vaccine lymphadenopathy
We retrieved 55 cases of C19-LAP with histological and/or cytological control and diagnosed as reactive lymphadenopathy (28 cases) or follicular hyperplasia (13 cases) [25-27, 30-34, 41-45, 47-56, 58, 59, 61-65, 68]. Kikuchi-Fujimoto Disease was reported in 6 cases while Tintle et al. reported Langerhans cell hyperplasia [45, 47, 48, 55, 62, 63]. Patil et al. described the case of atypical follicular hyperplasia with light chain-restricted germinal centers after COVID-19 booster [68]. The pooled analysis of 54 reports showed a mean age of 47.2±13.3 years old, with 80.0% (43/54) females. Fifteen (15/54, 28%) of these patients had no prior medical history, while six patients (6/54, 11%) had prior non-neoplastic medical history, including psoriatic arthritis in Haven et al., allergic asthma in Kaya et al., steroid-dependent minimal-change renal disease in Al Soub et al., and diabetes mellitus and hypertension in Tan HM et al. [45, 49, 50, 62]. When reported, most cases of lymphadenopathy occurred on the same side of the vaccination site, with contra-laterality reported in four cases (4/54, 7%) [31, 33, 44, 47]. The most common site of lymphadenopathy was the axillary region (28/55, 51%), followed by the clavicular (13/55, 24%) and cervical regions (6/55, 11%). The most reported associated symptoms included fever, pain. The mean dimension of lymph node reported was 21.3±10.9 mm. Ultrasound features were lymph node enlargement, cortical thickening, hypoechogenic areas, lost or partially detectable hilum and ill-defined borders [77]. Cases of KFD were reported in significantly younger patients with a mean age of 26.5±10.8 than those diagnosed with reactive lymphadenopathy (52.5±14.2 years old) and follicular hyperplasia (38.8±13.5 years old). The largest dimension of lymph node did not differ significantly amongst these three diagnoses (reactive lymphadenopathy: 24.5±12.7 mm, follicular hyperplasia: 14.3±3.3 mm, KFD: 20.4±7.9 mm). In the histopathological reports of C19-LAP, a case of Langerhans cell hyperplasia and a case of atypical follicular hyperplasia with light chain-restricted germinal centers have been described. KFD has been reported in six cases [45, 47, 48, 55, 62, 63, 68].
As already postulated for some cases of autoimmune diseases, the immunologic hyperstimulation or the hyperreactivity caused by RNA vaccines might be the reason why certain patients develop marked hyperplasia of germinal centers and pseudo-clonality [85]. In 26 out of 55 cases (47,3%), patients underwent imaging study during staging of a new diagnosed neoplastic disease or during cancer follow-up. In this kind of patients, chances of detecting subclinical lymphadenopathy as an incidental finding are increased. Moreover, it’s not uncommon that reactive lymphadenopathy may show pathological or suspicious features at imaging [86, 87]. Because of this, many patients undergo surgical lymph nodes excision for actionable diagnosis, raising the already high number of invasive medical procedures they have to endure, beyond the cost of the whole diagnostic process. In these cases, LN-FNAC and LN-CNB may represent the right solution. Unfortunately, LN-FNAC is not universally accepted as a diagnostic tool whereas it has been useful in evaluating malignant processes in cases in which surgical excisions were difficult to perform [25, 28, 82, 83, 88-94]. Moreover, LN-FNAC has been useful in the management of oncological patients, through the possibility of selecting patients who will truly benefit from CNB and, eventually, surgical excision.
CONCLUSIONS
SLDI and C19-LAP have represented a diagnostic dilemma and a clinical problem, especially in oncologic patients, which have often faced an invasive diagnostic approach. Because of the difficulties related to a surgical excision during the pandemic, CNB and FNAC, especially when combined with ROSE, have represented safe, cost-effective and accurate diagnostic tools, saving many patients an unnecessary surgical excision.
Conflict of interest
The authors have no conflict of interests related to this publication.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
[1] Albers JR, Brown JB, Charkowick SV, Ram N, Klocksieben FA, Kumar A. Comparative benefits and risks associated with currently authorized COVID-19 Vaccines. Vaccines (Basel). 2022; 10: 2065.
[2] Son S, Lee K. Development of mRNA Vaccines/Therapeutics and their delivery system. Mol Cells. 2023; 46: 41-47.
[3] Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. Brit Med J. 2021; 373: n1088.
[4] Ruiz-Fresneda MA, Ruiz-Pérez R, Ruiz-Fresneda C, Jiménez-Contreras E. Differences in global scientific production between new mRNA and conventional vaccines against COVID-19. Environ Sci Pollut Res Int. 2022; 29: 57054-57066.
[5] Medeiros LJ. Ioachim’s Lymph Node Pathology, 5th Ed. Lippincott Williams & Wilkins, 2022.
[6] Monaco SE, Khalbuss WE, Pantanowitz L. Benign non-infectious causes of lymphadenopathy: A review of cytomorphology and differential diagnosis. Diagn Cytopathol. 2012; 40: 925-938.
[7] Toy H, Karasoy D, Keser M. Lymphadenitis caused by H1N1 vaccination: case report. Vaccine. 2010; 28: 2158-2160.
[8] Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008; 28: 1194-1197.
[9] Pereira MP, Flores P, Neto AS. Neck and supraclavicular lymphadenopathy secondary to 9-valent human papillomavirus vaccination. BMJ Case Rep. 2019; 12(11): e231582.
[10] Atalar H, Sarifakioglu E, Dener C, Yanik B, Koktener A, Bayrak R. Cutaneous lymphoid hyperplasia and reactive lymphadenopathy induced by hepatitis B vaccination. Eur J Dermatol. 2008; 18: 188-189.
[11] Watanabe T, Hashidate H, Hirayama Y, Iinuma Y. Kikuchi-Fujimoto disease following vaccination against human papilloma virus infection and Japanese encephalitis. Eur J Pediatr. 2012; 171: 1409-1411.
[12] Sumaya CV, Cherry JD, Gohd R. Exaggerated antibody response following rubella vaccination in a child with sinus histiocytosis with massive lymphadenopathy. J Pediatr. 1976; 89: 81-83.
[13] Hartsock RJ. Postvaccinial lymphadenitis. Hyperplasia of lymphoid tissue that simulates malignant lymphomas. Cancer. 1968; 21: 632-649.
[14] Chua TH, Takano A. Pathological Findings in COVID-19 and Non-COVID-19 Vaccine-Associated Lymphadenopathy: A Systematic Review. J Clin Med. 2022; 11(21): 6290.
[15] White CK, Al-Saleem T, Skarbnik AP, Smith MR. Tetanus toxoid reactive lymphadenopathy masquerading as T-cell lymphoma. Future Oncol. 2012; 8: 631-634.
[16] Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N Engl J Med. 2021; 384: 1576-1577.
[17] Hartsock RJ. Postvaccinal lymphadenitis. Hyperplasia of lymphoid tissue that simulates malignant lymphomas. Cancer. 1968; 21: 632-649.
[18] Aelami MH, Alborzi A, Pouladfar G, Geramizadeh B, Pourabbas B, Mardaneh J. Post-vaccination disseminated bacillus Calmette Guerin infection among children in Southern Iran. Jundishapur J Microbiol. 2015; 8: e25663.
[19] Dotlic S, Vranic S, Jakovljevic G, Ilic I, Kardum-Paro MM, Dojcinov SD. Neonatal hyperimmune T-cell reaction mimicking T-cell non-Hodgkin’s lymphoma following BCG and hepatitis B co-vaccination. Virchows Arch. 2012; 461: 601-605
[20] Watanabe T, Hashidate H, Hirayama Y, Iinuma Y. Kikuchi-Fujimoto disease following vaccination against human papilloma virus infection and Japanese encephalitis. Eur J Pediatr. 2012; 171: 1409-1411.
[21] White CK, Al-Saleem T, Skarbnik AP, Smith MR. Tetanus toxoid reactive lymphadenopathy masquerading as T-cell lymphoma. Future Oncol. 2012; 8: 631-634.
[22] Yoshikawa T, Miki S, Nakao T, Koshino S, Hayashi N, Abe O. Axillary lymphadenopathy after Pfizer-BioNTech and Moderna COVID-19 vaccination: MRI Evaluation. Radiology. 2023; 306: 270-278.
[23] Center for Disease Control and Prevention. U.S. COVID-19 Vaccine Product Information, 2021. Available [Google Scholar].
[24] Pfizer Inc. Pfizer-BioNTech COVID-19 Vaccine EUA Fact Sheet for Recipients and Caregivers [Internet]. FDA; 2021. Available at https://www.fda.gov/media/144414/download.
[25] Caputo A, Caleo A, Cozzolino I, Zeppa P, Ciancia G, Ciliberti V. COVID-19 post-vaccination lymphadenopathy: A review of the use of fine needle aspiration cytology. Cytopathology. 2023; 34(5): 423-432.
[26] Gullotti DM, Lipson EJ, Fishman EK, Rowe SP. Acute axillary lymphadenopathy detected shortly after COVID-19 vaccination found to be due to newly diagnosed metastatic melanoma. Radiol Case Rep. 2022; 17: 878-880.
[27] Aalberg JJ, Collins, TCP, Dobrow EM. Axillary lymphadenopathy in a renal cell carcinoma patient after COVID-19 vaccination. Radiol. Case Rep. 2021; 16: 2164-2167.
[28] Kang ES, Kim MY. Bilateral cervical lymphadenopathy after mRNA COVID-19 vaccination on oral squamous cell carcinoma patient: a case report. Diagnostics (Basel). 2022; 12(7): 1518.
[29] Faermann R., Nissan N., Halshtok-Neiman O. et al. COVID-19 Vaccination induced lymphadenopathy in a specialized breast imaging clinic in Israel: Analysis of 163 cases. Acad Radiol. 2021; 28: 1191-1197.
[30] Ashoor A, Shephard J, Lissidini G, Nicosia L. Axillary adenopathy in patients with recent COVID-19 vaccination: a new diagnostic dilemma. Korean J Radiol. 2021; 22: 2124-2126.
[31] Eifer M, Tau N, Alhoubani Y, et al. COVID-19 mRNA vaccination: age and immune status and its association with axillary lymph node PET/CT uptake. J Nucl Med. 2021; 63: 134-139.
[32] Giorgis S, Garlaschi A, Brunetti N, et al. Axillary adenopathy after COVID-19 vaccine in patients undergoing breast ultrasound. J Ultrason. 2021; 21(87): e361-e364.
[33] Tripathy S, Alvarez N, Jaiswal S, et al. Hypermetabolic lymphadenopathy following the administration of COVID-19 vaccine and immunotherapy in a lung cancer patient: a case report. J Med Case Rep. 2022; 16(1): 445.
[34] Dirven I, Bravenboer B, Raeymaeckers S, Andreescu CE. Lymphadenopathy after COVID-19 vaccination in patients with endocrine cancer: two case reports. Endocrinol Diabetes Metab Case Rep. 2022; 2022: 22-0258.
[35] Landete E, Gómez-Fernández I, González-Gascón-Y-Marín I, et al. Hypermetabolic abdominal and cervical lymph nodes mimicking Hodgkin lymphoma relapse on FDG PET/CT after adenovirus-vectored COVID-19 vaccine. Hum Vaccin Immunother. 2021; 17: 5129-5132.
[36] Cohen D, Hazut Krauthammer S, Cohen YC, et al. Correlation between BNT162b2 mRNA COVID-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur J Nucl Med Mol Imaging. 2021; 48: 3540-3549.
[37] Abou-Foul AK, Ross E, Abou-Foul M, George AP. Cervical lymphadenopathy following coronavirus disease 2019 vaccine: clinical characteristics and implications for head and neck cancer services. J Laryngol Otol. 2021; 135: 1025-1030.
[38] Caputo A, Ciliberti V, D’Antonio A, et al. Real-world experience with the Sydney System on 1458 cases of lymph node fine needle aspiration cytology. Cytopathology. 2022; 33: 166-175.
[39] Uzun E, Erkilic S. Diagnostic accuracy of Thinprep® in cervical lymph node aspiration: Assessment according to the Sydney system. Diagn Cytopathol. 2022; 50: 253-262.
[40] Cozzolino I, Varone V, Picardi M, et al. CD10, BCL6, and MUM1 expression in diffuse large B-cell lymphoma on FNA samples. Cancer Cytopathol. 2016; 124: 135-143.
[41] Yu Q, Jiang W, Chen N, et al. Misdiagnosis of Reactive Lymphadenopathy Remotely After COVID-19 Vaccination: A Case Report and Literature Review. Front Immunol. 2022; 13: 875637.
[42] García-Molina F, Cegarra-Navarro MF, Andrade-Gonzales RJ, Martinez-Díaz F. Cytologic and histologic features of COVID-19 post-vaccination lymphadenopathy. Cytojournal. 2021; 18: 34.
[43] Tan NJH, Tay KXJ, Wong SBJ, Nga ME. COVID-19 post-vaccination lymphadenopathy: Report of cytological findings from fine needle aspiration biopsy. Diagn Cytopathol. 2021; 49: 467-470.
[44] Hagen C, Nowack M, Messerli M, Saro F, Mangold F, Bode PK. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med. Wkly. 2021; 151: w20557.
[45] Tan HM, Hue SS, Wee A, See KC. Kikuchi-Fujimoto Disease Post COVID-19 Vaccination: case report and review of literature. Vaccines (Basel). 2021; 9(11): 1251.
[46] Saito Y, Suwa Y, Kaneko Y, Tsujiwaki M, Odagawa Y. Kikuchi-Fujimoto Disease Following COVID-19 Infection in a 7-Year-Old Girl: A Case Report and Literature Review. Cureus. 2022; 14(7): e26540.
[47] Betancur V, Net J, Chapman J, Yepes M. Kikuchi-Fujimoto-like lymphadenopathy following COVID-19 vaccine: diagnosis and management. BMJ Case Rep. 2022; 15 (12): e252030.
[48] Daghri S, Belmoufid N, Rami A, Al Bouzidi A, Bouanani N. Kikuchi-Fujimoto’s Disease or Histiocytic Necrotizing Lymphadenitis Following mRNA COVID-19 Vaccination: A Rare Case. Cureus. 2022; 14(4): e24155.
[49] Kaya A, Kaya SY, Abul A, Fener N, Can A, Mert A. Eosinophilic lymph node abscesses following a COVID-19 vaccination: A case report. J Natl Med Assoc. 2023; S0027-9684(23)00003-2.
[50] Heaven CL, Barber L, Ahmadi O, Selvarajah K, Shetty S. COVID-19 vaccine associated cervical lymphadenopathy: a case series. ANZ J Surg. 2022; 92: 2286-22291.
[51] Cardoso F, Reis A, Osório C, Scigliano H, Yu Nora M. A Case of Cervical Lymphadenopathy After Vaccination Against COVID-19. Cureus. 2021; 13: e15050.
[52] Fernández-Prada M, Rivero-Calle I, Calvache-González A, Martinón-Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill. 2021; 6(10): 2100193.
[53] Yoshimoto N, Takura K, Yanagi A, et al. Axillary lymph node swelling mimicking breast cancer metastasis after COVID-19 vaccination: a japanese case report and literature review. In Vivo. 2022; 36: 1041-1046.
[54] Gogia P, Tanni F, Coca-Guzman J, Chen N, Huang Y. Case report: A rare case of Rosai-Dorfman-Destombes disease after the COVID-19 infection. Front Med (Lausanne). 2022; 9: 1073767.
[55] Ikeda K, Kakehi E, Adachi S, Kotani K. Kikuchi-Fujimoto disease following SARS-CoV-2 vaccination. BMJ Case Rep. 2022; 15(11): e250601.
[56] Placke JM, Reis H, Hadaschik E, et al. Coronavirus disease 2019 vaccine mimics lymph node metastases in patients undergoing skin cancer follow-up: A monocentre study. Eur J Cancer. 2021; 154: 167-174.
[57] Felices-Farias JM, Martínez-Martínez JF, Guzmán-Aroca F. Unusual lymphadenopathies secondary to the BNT162b2 mRNA COVID-19 vaccine. Med. Clin. 2021; 158: 343.
[58] Ganga K, Solyar AY, Ganga R. Massive Cervical Lymphadenopathy Post-COVID-19 Vaccination. Ear Nose Throat J. 2021 Oct 2:1455613211048984.
[59] Kado S, Kamiya K, Iwabuchi S, Kajii E, Ohtsuki M. Unilateral lymphadenopathy associated with COVID-19 vaccination. J Cutan Immunol Allergy 2021; 5: 100-101.
[60] Larkin K, Sharma A, Drachtman R, Salaru G. Supraclavicular lymphadenopathy after COVID-19 vaccination. Pediatr Blood Cancer. 2022; 69(5): e29516.
[61] Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: Diagnostic dilemma in oncologic Patients. Radiology. 2021; 300: E296-E300.
[62] Al Soub H, Ibrahim W, Al Maslamani M, Ali GA, Ummer W. Kikuchi-Fujimoto disease following SARS CoV2 vaccination: Case report. ID Cases. 2021; 25: e01253.
[63] Tintle S, Chen M. Lymphadenopathy with florid lymphoid and Langerhans cell hyperplasia and hemophagocytosis mimicking lymphoma after COVID-19 mRNA vaccination. E J Haem. 2021; 2: 845-847.
[64] Lim J, Lee SA, Khil EK, Byeon SJ, Kang HJ, Choi JA. COVID-19 vaccine-related axillary lymphadenopathy in breast cancer patients: Case series with a review of literature. Semin. Oncol. 2021; 48: 283-291.
[65] Trikannad A, Vellanki S, Kunapareddy G. Mediastinal Lymphadenopathy after COVID-19 vaccine: staging dilemma in oncology patients. Chest. 2021; 160: A1460.
[66] Robinson KA, Maimone S, Gococo-Benore DA, Li Z, Advani PP, Chumsri S. Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination. JAMA Oncol. 2021; 7: 1395-1397.
[67] Chua TH, Takano A. Pathological Findings in COVID-19 and Non-COVID-19 Vaccine-Associated Lymphadenopathy: A Systematic Review. J Clin Med. 2022; 11(21): 6290.
[68] Patil A, Swerdlow SH, Lossos IS, Chapman JR. Atypical follicular hyperplasia with light chain-restricted germinal centers after COVID-19 booster: a diagnostic pitfall. Virchows Arch. 2022; 13: 1-6.
[69] Nappi E, De Santis M, Paoletti G, et al. New Onset of eosinophilic granulomatosis with polyangiitis following mRNA-Based COVID-19 vaccine. Vaccines (Basel). 2022; 10(5): 716.
[70] Stimson L, Stitson R, Bahhadi-Hardo M, Renaudon-Smith E. COVID-19 associated Kikuchi-Fujimoto disease. Br J Haematol. 2021; 192(5): e124-e126.
[71] Akman B, Kaya AT. Effects of nonsteroidal anti-inflammatory drugs on ultrasound findings of mRNA COVID-19 vaccine-related lymphadenopathy. J Clin Ultrasound. 2022: 10.1002/ jcu.23390.
[72] Wong FC, Martiniova L, Masrani A, Ravizzini GC. 18F-Fluciclovine-Avid Reactive axillary lymph nodes after COVID-19 vaccination. Clin Nucl Med. 2022; 47: 154-155.
[73] Su N, Wiefels C, Klein R, Zeng W, Abbaspour F. Intensity of hypermetabolic axillary lymph nodes in oncologic patients in relation to timeline following COVID-19 vaccination. J Med Imaging Radiat Sci. 2022; 53: 219-225.
[74] Sahoo SS, Kaur N, Kaur A, Garg S. Lymphadenopathy subsequent to Covishield (ChAdOx1 nCoV-19) Corona virus vaccine: ultrasound findings and clinical implications. Ther Adv Vaccines Immunother. 2022 Sep 17; 10: 25151355221124018.
[75] Treglia G, Cuzzocrea M, Giovanella L, Elzi L, Muoio B. Prevalence and Significance of Hypermetabolic Lymph Nodes Detected by 2-[(18)F]FDG PET/CT after COVID-19 Vaccination: A Systematic Review and a Meta-Analysis. Pharmaceuticals. 2021; 14: 762.
[76] McIntosh LJ, Bankier AA, Vijayaraghavan GR, Licho R, Rosen MP. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol. 2021; 217: 975-983.
[77] Kim B, Park Y, Kim EK, Lee SH. Supraclavicular lymphadenopathy after COVID-19 vaccination in Korea: Serial follow-up using ultrasonography. Clin. Imaging. 2021; 79: 201-203.
[78] Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021; 21: 475-484.
[79] Bettini E, Locci M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines. 2021; 9: 147.
[80] Igual-Rouilleault AC, Soriano I, Quan PL, Fernandez-Montero A, Elizalde A, Pina L. Unilateral axillary adenopathy induced by COVID-19 vaccine: US follow-up evaluation. Eur Radiol. 2021; 1-8: 3199-3206.
[81] Tu W, Gierada DS, Joe BN. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Imaging Cancer. 2021; 3: e210038.
[82] Aelami MH, Alborzi A, Pouladfar G, Geramizadeh B, Pourabbas B, Mardaneh J. Post-Vaccination Disseminated Bacillus Calmette Guerin Infection Among Children in Southern Iran. Jundishapur J Microbiol. 2015; 8(11): e25663.
[83] Podugu A, Kobe M. Kikuchi-Fujimoto Disease (KFD): A Rare Cause of Fever and Lymphadenopathy Following Influenza Vaccination. Chest. 2013, 144, 230A.
[84] Park JY, Yi SY. Rare case of contralateral supraclavicular lymphadenopathy after COVID-19 vaccination: Computed tomography and ultrasonography findings. Radiol Case Rep. 2021; 16:3879-3881. Erratum in: Radiol Case Rep. 2022; 18:730-731.
[85] D’Ardia A, Caputo A, Fumo R, et al. Advanced non-small cell lung cancer: Rapid evaluation of EGFR status on fine-needle cytology samples using Idylla. Pathol Res Pract. 2021; 224: 153547.
[86] Kurtoğlu E, Göçer M. Clinical significance of increased FDG uptake in the Waldeyer ring and the nasopharynx region identified by pet-ct in postchemotherapy follow-up in patients with lymphoma: when should we perform biopsy? Clin Lymphoma Myeloma Leuk. 2020; 20(12): 830-835.
[87] Loh Z, Hawkes EA, Chionh F, Azad A, Chong G. Use of Ultrasonography facilitates noninvasive evaluation of lymphadenopathy in a lymph node diagnostic clinic. Clin Lymphoma Myeloma Leuk. 2021; 21(2): e179-e184.
[88] Vigliar E, Caleo A, Vitale M, Di Crescenzo V, Garzi A, Zeppa P. Early cytological diagnosis of extranodal stage I, primary thyroid non-Hodgkin lymphoma in elderly patients. Report of two cases and review of the literature. BMC Surg. 2013; 13 (Suppl. 2): S49.
[89] Cozzolino I, Vigliar E, Sosa Fernandez LV, et al. Non lymphomatous clonal B-Cell populations in enlarged lymph nodes in acquired immunodeficiency syndrome. Infez Med. 2012; 20 (Suppl. 2): 35-42.
[90] Tana C, Donatiello I, Caputo A, et al. Cytological features, histopathology and differential diagnosis of sarcoidosis. Cells. 2021; 11(1): 59.
[91] Ciliberti V, Maffei E, D’Ardia A, et al. Combined fine needle aspiration cytology and core needle biopsy in the same setting: A two-years’ experience. Cytopathology. 2024; 35(1): 78-91.
[92] Vigliar E, Cozzolino I, Fernandez LV, et al. Fine-needle cytology and flow cytometry assessment of reactive and lymphoproliferative processes of the breast. Acta Cytol. 2012; 56(2): 130-138.
[93] Cozzolino I, Zeppa R, Zeppa P. Lymph nodal Merkel cell carcinoma: primary tumor or metastasis from unknown primary site? J Cutan Pathol. 2011; 38(10): 836-837.
[94] Cozzolino I, Vigliar E, Todaro P et al. Fine needle aspiration cytology of lymphoproliferative lesions of the oral cavity. Cytopathology. 2014; 25(4): 241-249.