Le Infezioni in Medicina, n. 4, 575-582, 2023
doi: 10.53854/liim-3104-17
CASE REPORTS
Clinical utility of intrabronchial antifungal instillation in a complicated case of chronic pulmonary aspergillosis: case report and systematic review of literature
Sreyas Sharma, Rohit Kumar, Pranav Ish, Mahendran AJ, Neeraj Kumar Gupta, Nitesh Gupta, Manu Madan
Department of Pulmonary, Critical Care and Sleep Medicine, VMMC and Safdarjung Hospital, New Delhi, India
Article received 9 August 2023; accepted 11 October 2023
Corresponding author
Manu Madan
E-mail: manu.madan100@gmail.com
SummaRY
Chronic pulmonary aspergillosis (CPA) is a progressive, debilitating clinical condition associated with significant morbidity. Surgery is the mainstay of treatment for life-threatening hemoptysis in symptomatic patients with simple aspergillomas. However, in patients with chronic cavitary pulmonary aspergillosis, surgical removal of aspergillomas is fraught with difficulty due to debilitating nature of the illness. Here we present a case showcasing the utility of intrabronchial voriconazole instillation in controlling hemoptysis in a patient unfit for surgery followed by systematic review of literature involving 11 clinical studies after screening a total of 5572 studies from PubMed and Google Scholar database. Data gathered from these studies addresses the concerns regarding the efficacy, safety of the procedure as well as draws attention regarding several lacunae in our existing knowledge. A 53-year-old male with chronic pulmonary aspergillosis who had recurrent episodes of hemoptysis despite bronchial artery embolization and was unfit for surgery due to limited lung reserve, patient underwent single session of intrabronchial voriconazole instillation which resulted in dramatic symptomatic and radiological improvement. Intrabronchial antifungal instillation may be a safe and effective option for hemoptysis control in patients with chronic pulmonary aspergillosis.
Key words: Chronic pulmonary aspergillosis, Chronic cavitary pulmonary aspergillosis, Bronchial artery embolization, Antifungals.
INTRODUCTION
Chronic pulmonary aspergillosis (CPA) is a progressive, debilitating clinical condition associated with significant morbidity and several difficulties in diagnosis and management. CPA is further divided into several subtypes, including aspergillus nodule, single aspergilloma, chronic cavitary pulmonary aspergillosis (CCPA), chronic fibrosing pulmonary aspergillosis (CFPA) and subacute invasive pulmonary aspergillosis. Surgery is the mainstay of treatment for symptomatic patients with simple aspergillomas in order to treat life-threatening hemoptysis [1-3]. It is generally well-tolerated in patients with a single aspergilloma, with a mortality rate of <1% in multiple contemporary studies [4-6]. However, in patients with chronic cavitary pulmonary aspergillosis, surgical removal of aspergillomas is fraught with difficulty because of the very vascular, adherent pleura and because the residual pleural space may become infected with Aspergillus species, leading to Aspergillus empyema and/or a bronchopleural fistula [7]. Bronchial artery embolization (BAE) is an effective measure to control acute episode of hemoptysis in this sub population; however, BAE is not curative and recurrence rate is as high as 25% over the period of 1 year [8]. Other modalities such as percutaneous instillation of various antifungal agents like Amphotericin B, Ketoconazole and Voriconazole have been tried with various success rate in the past. Similarly, bronchoscopic instillation of antifungal agents into the affected lobe or segments for control of hemoptysis have been evaluated though in smaller studies [9-11]. In patients who are not candidates for surgical resection, bronchoscopic antifungal instillation may be an effective option. Here we present a case highlighting the utility of bronchoscopic Voriconazole instillation in a complicated case of chronic pulmonary aspergillosis.
CASE REPORT
A 53-year-old, male, smoker, presented to the outpatient department of chest clinic with complaints of cough for the last 11 years which was associated with mucopurulent expectoration since last 4 months and multiple episodes of hemoptysis (about 100-150 ml per episode) in the past 1 month. There was progressive shortness of breath for the last 6 years, which had worsened further to MMRC (Modified Medical Research Council dyspnea scale) grade IV over last 10 days. The patient also complained of low-grade fever, decreased appetite, and loss of weight in the last 4 months. He had a history of being treated for pulmonary tuberculosis 30 years back and had similar episodes of hemoptysis 1 year back for which he had undergone BAE at that time. On evaluation of his previous medical records, the patient had multiple Chest x-rays, which on serial examination revealed a progressive increase in size of left upper lobe cavitary lesion along with pericavitary infiltrates. CT scan of chest was done at the time of presentation to our department, which revealed left upper lobe thick-walled cavitary lesion with peri-cavitary consolidation and aspergilloma in left upper lobe with adjacent pleural thickening along with bilateral bronchiectasis, and bilateral centrilobular emphysematous changes (Figure 1). Flexible fibreoptic bronchoscopy was done which revealed thick purulent secretions in bilateral upper lobe bronchus. Mycobacterium tuberculosis and atypical mycobacterial infections were ruled out based on negative culture reports in bronchoalveolar lavage sample. Fungal culture of bronchoalveolar lavage grew Aspergillus fumigatus. Galactomannan levels in bronchoalveolar lavage and serum were 2.8 and 1.7 respectively. Serum specific-IgG against Aspergillus fumigatus was elevated (32 mgA/L). On fulfilling clinical, radiologic, and serological criteria, patient was diagnosed with chronic cavitary pulmonary aspergillosis. The patient was started on oral voriconazole but continued to have hemoptysis even after 2 months of voriconazole therapy, despite reaching adequate serum voriconazole levels (4.1 mg/L). Since the patient was unwilling to undergo for repeat BAE and was also not a candidate for surgery since it was a bilateral disease, a single session of bronchoscopic instillation of 400mg of voriconazole was done in the left upper lobe cavity as an adjunct to systemic therapy. The patient had transient bronchospasm and associated cough after the procedure which responded well to bronchodilators. Intrabronchial administration of voriconazole resulted in dramatic improvement and he did not experience further bouts of hemoptysis. A repeat CT chest was done one week after the procedure, which revealed dramatic resolution in the size of the aspergilloma (Figure 1C and 1D). The patient was subsequently discharged home on oral voriconazole. The patient is currently doing well even post 6 months after the procedure and is still being continued on oral voriconazole therapy. The patient has received a total of 8 months of oral voriconazole therapy.
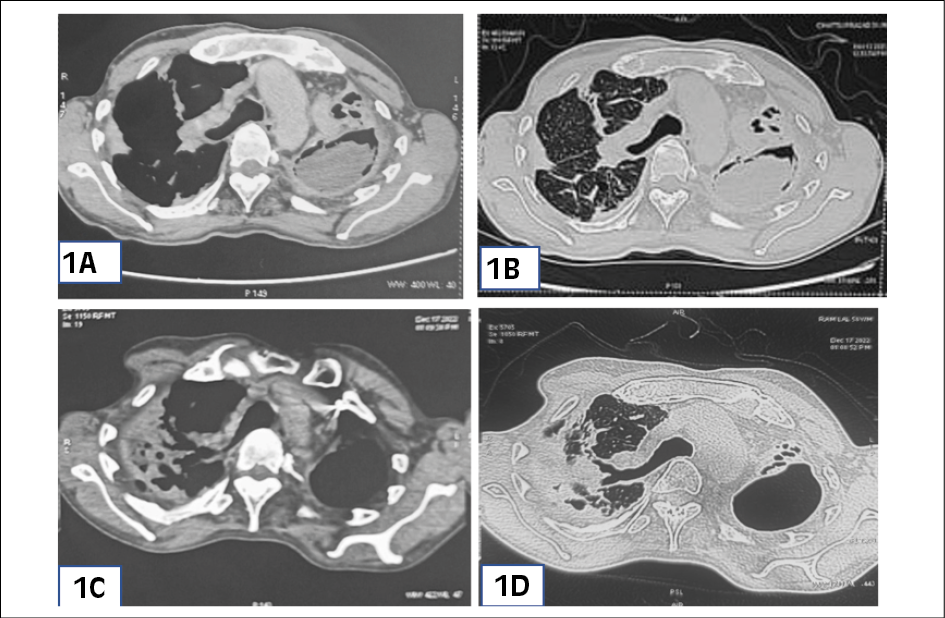
Figure 1 - (1A: Mediastinal window; 1B: Lung window) CT chest film (pre-procedure) showing left upper lobe thick walled cavitary lesion with pericavitary consolidation and aspergilloma with adjacent pleural thickening along with bilateral bronchiectasis with bilateral centrilobular emphysematous changes. (1C: Mediastinal Window: 1D: Lung Window) CT chest film (post-procedure) showing significant resolution in the size of aspergilloma in the left upper lobe.
The plausible mechanism of benefit from intrabronchial antifungal therapy is due to higher drug concentrations locally as a result of instillation directly into site of interest, which helps in reducing intra-cavitary fungal burden by causing mycelia death more reliably as compared to systemic therapy, as systemic therapy may have poor penetration into these cavities due to underlying fibrosis as well as variable pharmacokinetics of oral antifungal agents.
SYSTEMATIC REVIEW
We systematically searched the PubMed and Google scholar databases for the studies demonstrating the use of bronchoscopic guided antifungals in cases of chronic pulmonary aspergillosis using the key search terms (“endobronchial” OR “bronchoscopic”) AND (“voriconazole” OR “amphotericin” OR “antifungal”) AND (“aspergilloma” OR “CPA” OR “chronic pulmonary aspergillosis” OR “chronic cavitary pulmonary aspergillosis” OR “CCPA” OR “aspergillosis”). By utilizing our inclusion criteria, we enrolled clinical trials involving patients with CPA irrespective of the age group who had undergone endobronchial antifungal therapy, no language restrictions were adopted for enrolment of studies. Exclusion criteria included clinical trials involving patients with invasive pulmonary aspergillosis, allergic bronchopulmonary aspergillosis, fungal infections due to fungi other than aspergillus and trials involving patients with CPA who had not undergone endobronchial antifungal therapy. A total of 5572 searches (as of 23/06/2023) were screened for eligibility (5474 search results- Google scholar and 98 search results – Pubmed), 9 duplicate studies were excluded, and finally 11 studies (including 1 randomized controlled trial, 4 case series and 6 case reports) were selected for systematic review. Figure 2 shows flowchart used for article selection. Details of studies included are tabulated in Table 1.
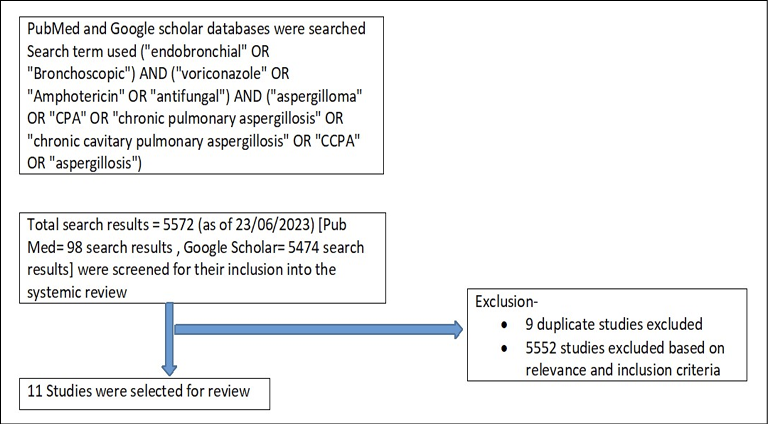
Figure 2 - Flowchart for article selection.
Table 1 - Studies demonstrating the use of endobronchial antifungal treatment.
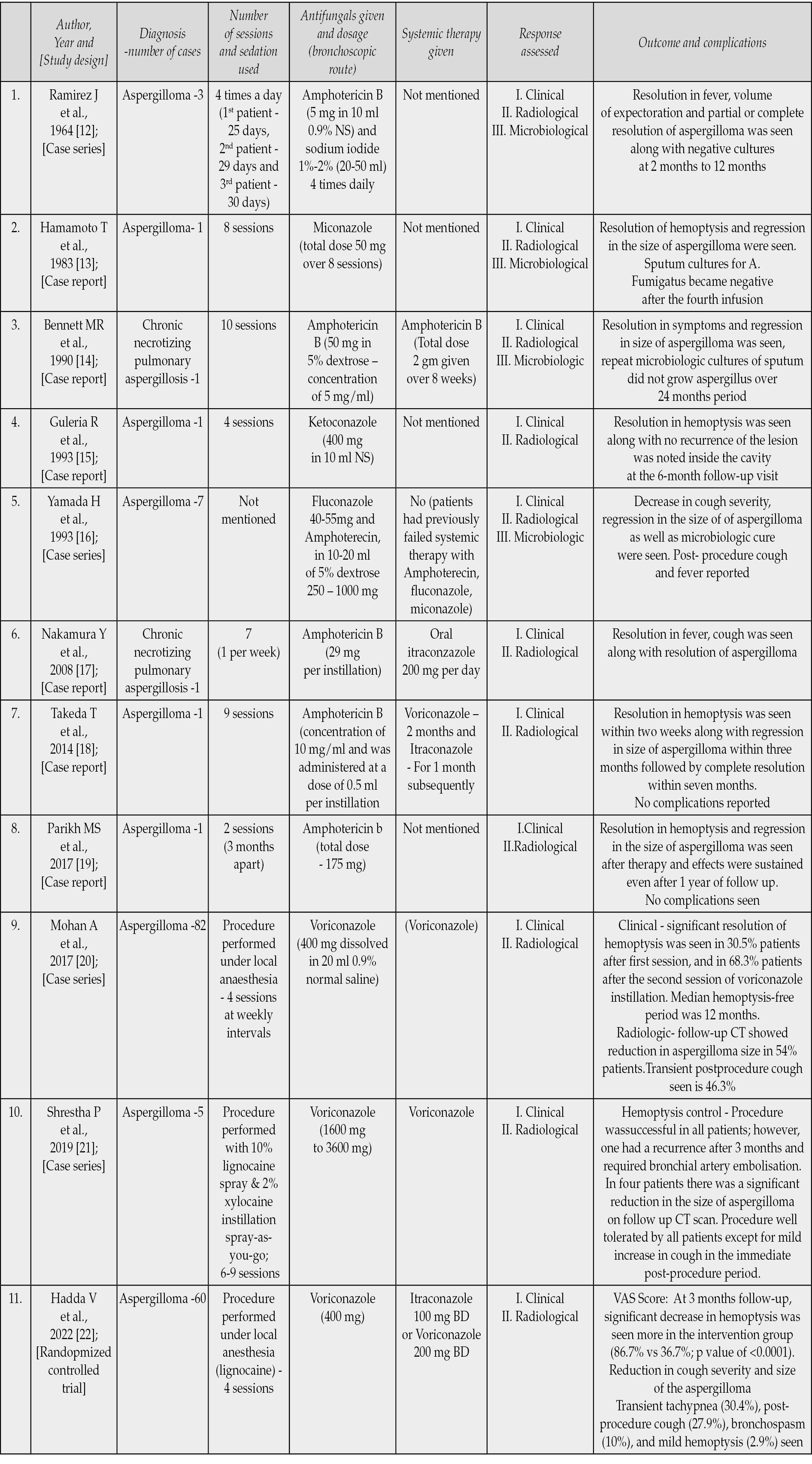
The first report of successful endobronchial treatment of 3 cases of aspergilloma with amphotericin B and sodium iodide was by Ramirez in 1964 [12]. Till date only one pilot randomized trial has been conducted by Hadda V et.al [22]. In this study, participants in the control arm were managed with medical therapy alone that consisted of antitussives and hemostatic agents (tranexamic acid or ethamsylate), or antifungal as prescribed by the treating physician whereas in the intervention arm, participants underwent bronchoscopic instillation of 400 mg voriconazole, for 4 sessions, 1 week apart, in addition to medical therapy. The primary objective of this study was to compare the percentage of patients achieving reduction in hemoptysis severity as assessed on visual analogue scale (VAS) in intervention and control arm at 3 months. VAS was presented to the participants as a 100-mm horizontal line anchored with description of “no hemoptysis” at 0 mm and “the worst imaginable hemoptysis” at 100 mm point [23]. Hemoptysis reduction was defined as a decrease of hemoptysis by 10 or more points on VAS, as compared to baseline. They reported that at 3 months follow-up, significant decrease in hemoptysis was seen more in the intervention group as compared to control group (86.7% vs 36.7%; p value of <0.0001). Intervention group had statistically significant reduction in hemoptysis at the end of first week (73.3% vs. 36.7%; p=0.004) and fourth week (86.7% vs. 60%, p=0.019). Bronchoscopic instillation of voriconazole was also associated with reduction in cough severity and size of the aspergilloma. However, there was no benefit of this therapy in terms of requirement of hospitalization and BAE. They reported that most of the bronchoscopic sessions were uneventful. Out of total 240 bronchoscopic sessions, transient tachypnea (30.4%), post-procedure cough (27.9%), bronchospasm (10%), and mild hemoptysis (2.9%) being most common post-procedure complications. There were no major complications. This RCT although demonstrating a beneficial response from intrabronchial therapy but had some significant limitations as well. Although the latest guidelines recommend systemic azoles for the treatment of symptomatic chronic aspergillosis, only a few patients received systemic antifungals in either of the arms; and this constitutes a major limitation of the study [1]. Due to this reason this study cannot comment on role of intra-cavitary voriconazole in conjunction with systemic antifungals. Authors tried to minimize the spillage of drug, by positioning the patients in ipsilateral posture, which may not be the best way but it is an easy to perform technique, thus it constitutes another limitation.
Other than the above mentioned RCT, one of the largest case series of intracavitary voriconazole instillation for hemoptysis control in patients with pulmonary aspergilloma was reported by Mohan A et al. [20] demonstrating the utility of endobronchial voriconazole therapy. In their retrospective case series of 82 patients with pulmonary aspergilloma, they used four sessions of intrabronchial voriconazole (400 mg dissolved in 20 ml of 0.9% normal saline) at weekly intervals. The most common underlying etiology for pulmonary aspergilloma was post-tubercular sequelae (95.1%) in their study. The procedure successfully controlled hemoptysis in 78 (95.1%) patients. Of these, 25 patients (30.5%) achieved hemoptysis control after the first instillation itself, and 77 (93.9%) patients after the second session. Only 4 patients required rescue BAE or surgery following voriconazole treatment. Transient post-procedure cough seen in 46.3% patients was the commonest procedure-related adverse event, other than that bronchospasm requiring treatment (7.3%) and lower respiratory tract infections (6.1%) were also seen. Prior history of BAE and the baseline aspergilloma size were the potential risk factors for the recurrence of hemoptysis after the procedure. In view of scant literature regarding the optimum dose of voriconazole, we also used 400 mg of voriconazole instillation as described by Mohan A et al and Hadda V et.al in our patient [22, 24].
Apart from these two studies, multiple case reports and case series have highlighted the efficacy of antifungal instillations in achieving clinical as well as radiological resolution but there is a dearth of good quality studies and there are many lacunae in our existing knowledge. There has been no consensus regarding the optimal concentration, dosage of drug, frequency of sessions and duration of therapy in achieving best possible response. Similarly, there has been no consensus between the ideal mode of sedation/ analgesia (either general or local anaesthesia) to ensure better drug delivery and preventing spillage into other segments. It is expected in a procedure during general anaesthesia, that there is no cough; hence, the drug would remain in the segment for longer period and may have better efficacy. No studies have evaluated the role of endobronchial blockers either, which may theoretically result in higher local concentration. There has also been no consensus among studies regarding the method of assessing hemoptysis reduction. The quantification of hemoptysis is often difficult, and both over- and under-quantification of expectorated blood volume is common. No objective parameter was used in any of the above studies in assessing hemoptysis reduction except the VAS, which was used in the study by Hadda V et.al, which is not a very accurate method [22]. One of the other important limitations of this treatment modality is its invasive nature, which requires the procedure to be performed in either day care setting or after hospitalization. In most of the reported studies, multiple sessions of antifungal instillations were administered, thus increasing the risk of minor adverse events. These factors can probably hinder the acceptability of this treatment modality as a first line treatment approach among patients. So it is important to realise that this treatment modality should be considered when conservative management fails and should not be used empirically in all patients presenting initially with hemoptysis. Apart from CPA, similar treatment modalities have also been utilised in the management of patients with invasive pulmonary aspergillosis [24-27]. Future randomized controlled trials using larger sample size, addressing the current lacunae in our existing knowledge are needed, to devise an optimal method for antifungal instillation as well as to compare endobronchial antifungal therapy with the systemic therapy.
CONCLUSION
Intrabronchial voriconazole instillation may be a safe and effective option for hemoptysis control in patients with chronic pulmonary aspergillosis and can be considered as an adjunct to systemic therapy, or who are not candidates for surgical resection and bronchial artery embolization. Future studies are needed for answering some important questions such as the optimal dose, optimal frequency of sessions, total duration of therapy, optimal analgesia/ sedation policy and methods to ensure longer drug contact time and lesser spillage into other segments.
Conflicts of interests
The authors declare that they have no conflicts of interest in regard to current study.
Funding
The current study is not funded.
Availability of data and material
The clinical data and the study materials available from the corresponding author on reasonable request.
Patient consent
Written informed patient consent taken.
Authors’ contribution
MM, SS, RK, NG were involved in literature searching, planning, conducting, writing the original draft of manuscript, and editing. MM, PI, SS, RK, NG, NKG, AJM were involved in reviewing and editing the manuscript. All the authors have agreed with the submitted manuscript. PI is the guarantor for all.
REFERENCES
- Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016; 47: 45.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016; 63: e1.
- Moodley L, Pillay J, Dheda K. Aspergilloma and the surgeon. J Thorac Dis. 2014; 6: 202.
- Farid S, Mohamed S, Devbhandari M, et al. Results of surgery for chronic pulmonary Aspergillosis, optimal antifungal therapy and proposed high risk factors for recurrence-a National Centre’s experience. J Cardiothorac Surg. 2013; 8: 180.
- Lejay A, Falcoz PE, Santelmo N, et al. Surgery for aspergilloma: time trend towards improved results? Interact Cardiovasc Thorac Surg. 2011; 13: 392.
- Chen QK, Jiang GN, Ding JA. Surgical treatment for pulmonary aspergilloma: a 35-year experience in the Chinese population. Interact Cardiovasc Thorac Surg. 2012; 15: 77.
- Daly RC, Pairolero PC, Piehler JM, et al. Pulmonary aspergilloma. Results of surgical treatment. J Thorac Cardiovasc Surg. 1986; 92: 981.
- Shin BS, Jeon GS, Lee SA, Park MH. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2011; 15: 1093-1098.
- Ortiz de Saracho J, Perez-Rodriguez E, Zapatero J, et.al. Therapeutic alternatives in complicated nonsurgical pulmonary aspergillomas. Arch Bronconeumol. 1995; 31: 83-85.
- Klein JS, Fang K, Chang MC. Percutaneous transcatheter treatment of an intracavitary aspergilloma. Cardiovasc Intervent Radiol. 1993; 16: 321-324.
- Krakowka P, Traczyk K, Walczak J, et.al. Local treatment of aspergilloma of the lung with a paste containing nystatin or amphotericin B. Tubercle. 1970; 51: 184-91.
- Ramirez J. Pulmonary aspergilloma: endobronchial treatment. N Engl J Med. 1964; 271: 1281-1285.
- Hamamoto T, Watanabe K, Ikemoto H. Endobronchial miconazole for pulmonary aspergilloma. Ann Int Med. 1983; 98: 1030.
- Bennett MR, Weinbaum DL, Fiehler PC. Chronic necrotizing pulmonary aspergillosis treated by endobronchial amphotericin B. South Med J. 1990; 83: 829-832.
- Guleria R, Gupta D, Jindal SK. Treatment of pulmonary aspergilloma by endoscopic intracavitary instillation of ketoconazole. Chest. 1993; 103: 1301-1302.
- Yamada H, Kohno S, Koga H, et.al. Topical treatment of pulmonary aspergilloma by antifungals. Relationship between duration of the disease and efficacy of therapy. Chest. 1993; 103: 1421-1425.
- Nakamura Y, Shirai M, Hayakawa H, et.al. Chronic necrotizing pulmonary aspergillosis successfully diagnosed, treated, and followed by ultrathin bronchoscope. Mycoses. 2008; 51: 86-88.
- Takeda T, Itano H, Kakehashi R, et.al. Direct transbronchial administration of liposomal amphotericin B into a pulmonary aspergilloma. Respir Med Case Rep. 2014; 11: 7-11.
- Parikh MS, Seeley E, Nguyen-Tran E, Krishna G. Endobronchial ultrasound-guided transbronchial needle injection of liposomal amphotericin b for the treatment of symptomatic aspergilloma. J Bronchology Interv Pulmonol. 2017; 24: 330-333.
- Mohan A, Tiwari P, Madan K, et.al. Intrabronchial voriconazole is a safe and effective measure for hemoptysis control in pulmonary aspergilloma. J Bronchol Intervent Pulmonol. 2017; 24: 29-34.
- Shrestha P, Dhungana A. Bronchoscopic Voriconazole instillation in pulmonary aspergilloma: a single center experience. Nep Med J. 2019; 2: 173-176.
- Hadda V, Doddamani S, Mittal S, et.al. Efficacy of Intrabronchial voriconazole instillation for inoperable pulmonary aspergilloma: a pilot randomized controlled trial. Respiration. 2022; 101: 833-840.
- Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990; 13: 227–236.
- Canetti D, Cazzadori Ao, Adami I, et al. Aerosolized amphotericin B lipid complex and invasive pulmonary aspergillosis: a case report. Infez Med. 2015; 23 (1): 44-47.
- Tiseo G, Galfo V, Occhineri S, et al.Risk factors and outcomes of fungal superinfections in patients with severe COVID-19: an observational study from Pisa academic hospital. Infez Med. 2023; 31 (1): 55-61.
- Sav H, Atalay MA, Koc AN, et al. Utility of the Aspergillus galactomannan antigen testing for neutropenic paediatric patients. Infez Med. 2017; 25 (1): 38-44.
- Sindhu D, Jorwal P, Gupta N, et al. Clinical spectrum and outcome of hospitalized patients with invasive fungal infections: a prospective study from a medical ward/intensive care unit of a teaching hospital in North India. Infez Med. 2019; 27 (4): 398-402.