Le Infezioni in Medicina, n. 4, 517-532, 2023
doi: 10.53854/liim-3104-11
ORIGINAL ARTICLES
Etiological characterization of acute undifferentiated febrile illness in Apartadó and Villeta municipalities, Colombia, during COVID-19 pandemic
Carlos Ramiro Silva-Ramos1, Juliana Gil-Mora1, Cristian C. Serna-Rivera2, Heidy-C. Martínez Díaz1, Nicaela Restrepo-López2, Piedad Agudelo-Flórez3, Margarita Arboleda4, Francisco J. Díaz5, Álvaro A. Faccini-Martínez6,7,8, Marylin Hidalgo1, Peter C. Melby9,10, Patricia V. Aguilar10,11, Miguel M. Cabada9,10, Alberto Tobón-Castaño12, Juan David Rodas2, and members of the GIDRN – Global Infectious Diseases Research Network
1Grupo de Enfermedades Infecciosas, Departamento de Microbiología, Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá, Colombia;
2Grupo de Investigación en Ciencias Veterinarias Centauro, Universidad de Antioquia, Medellín, Colombia;
3Grupo de Investigación en Ciencias Básicas, Escuela de Graduados, Universidad CES, Medellín, Colombia;
4Instituto Colombiano de Medicina Tropical, Universidad CES, Medellín, Colombia;
5Grupo de Inmunovirología, Universidad de Antioquia, Medellin,Colombia;
6Servicio de Infectología, Hospital Militar Central, Bogotá, Colombia;
7Servicios y Asesorías en Infectología - SAI, Bogotá, Colombia;
8Facultad de Medicina, Universidad Militar Nueva Granada, Bogotà, Colombia;
9Division of Infectious Diseases, Department of Internal Medicine, University of Texas Medical Branch, Galveston, Texas, USA;
10Center for Tropical Diseases, University of Texas Medical Branch, Galveston, Texas, USA;
11Department of Pathology, University of Texas Medical Branch, Galveston, Texas, USA;
12Grupo de Malaria, Universidad de Antioquia, Medellín, Colombia
Article received 26 September 2023; accepted 2 November 2023
Corresponding authors
Carlos Ramiro Silva-Ramos
E-mail: cramiro-silva@javeriana.edu.co
Juan David Rodas
E-mail: jdavid.rodas@udea.edu.co
SummaRY
Background: Acute undifferentiated febrile illness (AUFI) is one of the leading causes of illness in tropical regions. Although malaria is the most important cause, other pathogens such as Dengue (DENV), Leptospira and recently, Coronavirus Disease 2019 (COVID-19) have gained importance. In Colombia, few studies aimed to identify the etiology of AUFI. Most of them performed in Apartadó and Villeta municipalities, identifying the active circulation of several pathogens. Thus, we conducted a cross-sectional study in these municipalities to characterize the etiologies of AUFI during COVID-19 pandemic.
Methods: An active surveillance was conducted between September and December 2021 in local hospitals of Apartadó and Villeta municipalities. Febrile patients were enrolled after voluntarily agreeing to participate in the study. Ten different etiologies were evaluated through direct, serological, molecular and rapid diagnostic methods.
Results: In Apartadó a confirmed etiology was found in 60% of subjects, DENV (25%) being the most frequent, followed by leptospirosis (16.7%), malaria (10%), COVID-19 (8.3%), spotted fever group (SFG) rickettsiosis (6.7%) and Chikungunya (1.7%). In Villeta, a specific etiology was confirmed in 55.4% of patients, of which SFG rickettsiosis (39.3%) was the most frequent, followed by leptospirosis (21.4%), DENV (3.6%) and malaria (1.8%). No cases due to Mayaro, Yellow Fever, Oropouche and Venezuelan Equine Encephalitis viruses were detected.
Conclusion: We confirm the relevance of dengue fever, leptospirosis, SFG rickettsiosis, COVID-19 and malaria as causes of AUFI in the municipality of Apartadó, and highlight the great importance of SFG rickettsiosis as the main cause of AUFI in the municipality of Villeta.
Keywords: Arbovirus, COVID-19, Leptospira, Malaria, Rickettsia.
INTRODUCTION
Acute undifferentiated febrile illness (AUFI) is a term used to define a fever of less than two weeks (fourteen days) of duration without any evident focus of infection on initial physical examination or in basic laboratory tests [1, 2]. However, some authors used the term AUFI to designate febrile cases of up to seven days, while febrile cases lasting 7 to 28 days or lasting more than 28 days are designated as “fever of intermediate duration” or “chronic febrile illness,” respectively [3, 4]. Furthermore, AUFI has been referred to using different names depending on the authors and the geographical region. Some of the most used names are “acute febrile illness”, “acute fever of unknown origin”, “short-term febrile illness”, “acute undifferentiated fever”, “undifferentiated tropical febrile illness”, “tropical fever” and “acute febrile syndrome” highlighting the difficulties in identifying a specific etiology for these short lived febrile illnesses in the tropics [5-10].
Although AUFI can be a self-limited or a short-term illness, it can also develop into a more severe, potentially lethal disease that requires prompt recognition. Early identification of specific causes of AUFI may help to initiate specific therapy or referral to a higher-level health center to avoid fatal outcomes or chronic complications [11, 12]. Nevertheless, managing AUFI still represents a great challenge due to the wide range of etiologies that present with fever and nonspecific signs and symptoms, the limited diagnostic resources in endemic regions, and the lack of epidemiological surveillance worldwide [11, 12]. Despite the efforts to develop clinical algorithms that allow healthcare personnel to narrow down the potential etiological diagnosis, making accurate clinical diagnosis without laboratory confirmation remains challenging [13].
In Latin America and the Caribbean, AUFI is one of the leading causes of medical consultation in tropical and subtropical resource-limited regions and is often associated with high morbidity and mortality [9]. Malaria is still considered an important cause of AUFI in most of these regions despite the progress towards its elimination. In approximately 21 countries in the region, malaria remains endemic with approximately 120 million people at risk of infection [14]. Currently, it is believed that the principal cause of AUFI are arboviral infections, including Dengue virus (DENV), which represents the most common etiology of febrile illness in the tropics, followed by Chikungunya virus (CHIKV) and Zika virus (ZIKV), at least during their emergence in 2013 and 2015, respectively [15]. Other etiologies, such as leptospirosis, are also considered important due to their widespread geographical distribution [9, 16]. Coronavirus Disease 2019 (COVID-19), the disease caused by the pandemic Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has also been linked as an etiology of AUFI in the tropics [17, 18]. However, there are several other etiologies of AUFI that are not commonly monitored by local surveillance systems such as rickettsiosis and other arboviral diseases (e.g., Mayaro fever, Oropouche fever) [19]. Therefore, little is known about their local epidemiology, and some of them are totally unknown to healthcare personnel [20].
Colombia is the second most diverse country on the planet with a wide range of altitudes and temperatures. Due to its proximity to the Equator, the climate in Colombia is mainly tropical and isothermal, with only rainy and dry seasons and limited weather variation throughout the year [21]. However, climate changes due to global warming is also impacting Colombia, leading to unusual temperatures [21, 22]. At least 85% of the country is composed of tropical and subtropical areas, which provide ideal ecosystems for multiple pathogens causing AUFI [23]. Currently, there are very few studies conducted in Colombia aimed at identifying the etiology of AUFI [8, 24-26]. While DENV represented the main etiology of this syndrome in several regions, Leptospira spp. was found to be one of the most frequent etiologies of AUFI, even more common than DENV in some other regions [25].
Two of the main endemic regions for AUFI in Colombia are the municipalities of Apartadó, department of Antioquia, and Villeta, department of Cundinamarca, in which previous studies have found active circulation of Leptospira, DENV and other pathogens [24, 25, 27, 28]. Nonetheless, in recent years, climate change, increased migration of international refugees, and the COVID-19 pandemic may have impacted the etiology of AUFI in these regions as described elsewhere. Therefore, we conducted a cross-sectional study from September to December 2021 in the Apartadó and Villeta municipalities to characterize the etiologies of AUFI.
PATIENTS AND METHODS
Study area
The present study was performed in the municipalities of Apartadó and Villeta (Figure 1), two recognized endemic regions for AUFI [24, 25].
Figure 1 - Map with the location of the municipalities where patient recruitment was done in the present study. The municipalities of Apartadó, Antioquia department, and Villeta, Cundinamarca department, are marked
with red.
Apartadó (7°52´40” N, 76°37´44” W) is the most developed municipality located in the northwestern region of the department of Antioquia, in an area commonly known as “Urabá antioqueño”, at 336 km from Medellin city (the capital of the department of Antioquia). Apartadó municipality comprises 57 villages distributed in a total area of 600 Km2 at an elevation of 25 meters above sea level (masl). The annual mean temperature ranges between 24-32°C and the relative humidity between 86-90%. The predominant economic activities in this municipality, are agriculture, mainly banana industry, followed by the production of cocoa, corn, rice, and cassava (https://www.apartado-antioquia.gov.co). According to the 2018 national population and housing census of the “National Administrative Department of Statistics - Departamento Administrativo Nacional de Estadística” (DANE), Apartadó has a total population of 113,462 inhabitants, of which 91,811 live in the urban area, 13,999 live in suburban settlements and 8,259 live in rural areas (https://www.dane.gov.co).
Villeta (5°00´46” N, 74°28´23” W), is a municipality located in Cundinamarca department in the province of Gualivá, at 84 km from Bogotá D.C. (the capital of Colombia). Villeta municipality comprises 22 villages distributed in a total area of 140 Km2 at an elevation of 850 masl. The annual mean temperature is 26°C and the relative humidity ranges between 80%-97%. Eco-tourism and agriculture are the predominant economic activities of the region, with sugarcane and “panela” production being the main economic activity in rural areas (www.villeta-cundinamarca.gov.co). According to the 2018 national population and housing census of the DANE, Villeta has a total population of 25,957 inhabitants, of which 17,751 live in the urban area, 743 live in suburban settlements and 7,463 live in rural areas (https://www.dane.gov.co).
Ethical aspects
The study protocol, the informed consent, and assent were approved by the ethics committee of Pontificia Universidad Javeriana and the Bioethics Committee of the Medical School at the University of Antioquia. Each of the recruited adult voluntarily signed the written informed consent before any study procedure was performed. For subjects less than 6-years-old and those in critical condition, signed informed consent was obtained from the parents or the legal guardians. In the case of subjects older than 6-years but younger than 18-years, an informed assent was obtained from the participant and the parents or legal guardians provided a written informed consent. All signed informed consent forms were kept in locked cabinets, and were not available to anyone but the principal investigators. The information obtained was deidentified by assigning a numeric code to each subject included in the study. The study procedures, management, conservation of biological specimens, and technical-administrative procedures adhere to health research regulations as stated in resolution 8430 of the Ministry of Health of Colombia from 1993 and declaration of Helsinki for ethical and medical research in human subjects.
Patients, samples, and data collection
The present study was part of a larger multisite, multi-country study of AUFI that used a common protocol backbone and data collection system. Results from the other studied sites and any aggregate results from the network will be reported later.
We conducted active surveillance between September and December of year 2021 in “Antonio Roldán Betancur” Hospital in Apartadó municipality and “Salazar de Villeta” Hospital in Villeta municipality to determine the etiology of AUFI. Patients were enrolled in the study after being evaluated in the emergency department for a febrile illness with no-specific focus of infection or diagnosis. Whole blood, serum, urine, and oropharyngeal swab samples were collected on enrollment (acute phase sample) and only serum was collected 15 to 30 days after the onset of symptoms (convalescent phase sample). Samples from Apartadó municipality were sent and stored in the “Laboratorio Centauro” of the “Universidad de Antioquia” in Medellin. Samples from Villeta municipality were sent and stored in the “Laboratorio de Bacteriología Especial” of the “Pontificia Universidad Javeriana” in Bogotá D.C. Clinical, demographic, and epidemiological data were also collected from each subject during the clinical evaluation and were entered in the Research Electronic Data Capture (REDCap) platform [29, 30].
Inclusion and exclusion criteria
Male and female patients older than 2-years who were evaluated in the emergency departments for an AUFI (without a clear source) of less than fourteen days of evolution were eligible for inclusion in the study. Fever, defined as a temperature >37.8 ˚C, should have been documented by the subject or by the health personnel before inclusion.
Exclusion criteria included:
1) subjects of less than 2-years-old,
2) subjects in which an identifiable source of infection was evident (e.g. otitis media, sinusitis, purulent pharyngitis, skin and soft tissue infection, urinary tract infection, pneumonia, dental abscess, osteomyelitis) to the healthcare provider, and
3) subjects who did not agree to voluntarily participate in the study or did not sign the informed consent.
Diagnostic methods
A total of ten different etiologies were evaluated through direct, serological, molecular and rapid diagnostic methods. These included SARS-CoV-2, malaria, DENV, CHIKV, Mayaro virus (MAYV), Yellow Fever virus (YFV), Oropouche virus (OROV), Venezuelan Equine Encephalitis virus (VEEV), Leptospira spp. and Spotted Fever Group (SFG) Rickettsia spp.
We used the GeneFinderTM COVID-19 Plus Real Amp kit for SARS-CoV-2 (ELITechGroup, France) for detection of SARS-CoV-2 viral genome in oropharyngeal swab samples processed in the “Instituto Colombiano de Medicina Tropical” in Apartadó municipality and the “Universidad de Los Andes” in Bogotá D.C.
We performed thick smear microscopy and the SD BIOLINE assay for Malaria Ag Pf/Pv rapid diagnostic test (RDT) (Standard Diagnostic Inc., Korea) to screen whole blood samples for malaria at each hospital. We tested positive samples with a malaria nested PCR (nPCR) using the primers rPLU1 and rPLU5 in the first screening step for genus detection, and rPLU3 and rPLU4 in the second round for species identification as previously described [31].
We used the SD BIOLINE™ Dengue Duo rapid test (Standard Diagnostic Inc., Korea) for detection of DENV NS1 antigen and IgG/IgM antibodies on acute phase serum samples at each hospital. Two trioplex real time PCR tests for the detection of arbovirus designed and validated at the University of Texas Medical Branch (Fernandez D et al, manuscript in preparation) were used in serum samples obtained during the acute phase of the illness. The first trioplex assays used primers and probes targeting a fragment of the following molecular targets: DENV 3’ noncoding region, MAYV structural polyprotein, and CHIKV non-structural polyprotein; and the second targeted a fragment of the following molecular targets: YFV 5’ noncoding region, OROV 5’ end of the S-segment, and VEEV non-structural polyprotein. The primers and probes sequences for DENV, YFV and OROV have already been published [32,33]. However, information regarding the assays for other viruses used in the trioplex PCR assays will be published elsewhere and are available to scientists upon request.
We used the PanbioTM Leptospira ELISA (Inverness Medical Innovations, Queensland, Australia) to detect Leptospira IgM on serum samples. We also performed a PCR targeting a 317-base pair fragment of the 16s rRNA gene of Leptospira spp. using the primers F16S (GGCGCGTCTTAAACATGCAAG) and R16S (GAGCAAGATTCTTAACTGCTGCC) [34] on serum and urine samples. Positive serum samples by either of these methods, were further analyzed by microscopic agglutination test (MAT) using nine different Leptospira serovars (Autumnalis, Ballum, Bratislava, Canicola, Grippotyphosa, Hardjo, Icterohaemorrhagiae, Pomona, Tarassovi).
We performed an indirect immunofluorescence assay (IFA) in acute and convalescent phase serum samples to detect SFG Rickettsia IgG antibodies using the Rickettsia Screen IFA IgG Antibody Kit (Fuller Laboratories, CA, USA).
Definitions of confirmed infections
Definitions for confirmed cases for all the pathogens tested follow the criteria established by the National Institute of Health of Colombia (INS) (www.ins.gov.co) and the Center for Disease Control and Prevention (CDC) from the United States of America (www.cdc.gov).
A confirmed case of COVID-19 was defined as the detection of SARS-CoV-2 RNA in qRT-PCR of oropharyngeal secretions. A malaria case was confirmed by the presence of blood parasites consistent with Plasmodium spp. on thick blood smears and/or the detection of P. falciparum/P. vivax antigens on an RDT, and/or the detection of Plasmodium DNA on a nPCR performed on a sample collected during the acute phase of AUFI. A DENV infection was confirmed by the detection of IgM antibodies against DENV along with the detection of DENV NS1 antigen, the detection of IgG antibodies against DENV along with the detection of DENV NS1 antigen, and/or detection of viral RNA through qRT-PCR in a serum sample collected during the acute phase. MAYV, CHIKV, YFV, OROV and VEEV infections were all confirmed by detection of viral RNA through qRT-PCR performed on a serum sample collected during the acute phase of the illness. Leptospirosis was confirmed by detection of IgM antibodies against Leptospira in acute and/or convalescent serum samples and/or detection of bacterial DNA through PCR and confirmation by sequencing. Serovar identification was performed by documenting a fourfold increase in one or more serovar specific antibody titers using MAT on serum samples. Rickettsia infections were confirmed by a fourfold increase in IgG antibody titers between acute and convalescent serum samples through IFA; following expert recommendations, clinical compatible cases in which at least one of the samples had IgG antibody titers ≥1:256 were also considered positive [35].
Statistical analysis
Frequency tables were made for demographic, epidemiological, etiological, and clinical categorical variables. We used means ± standard deviations (±SD) and medians with interquartile ranges (IQR) to summarize variables with normal and non-normal distributions, respectively. Pearson’s chi-squared test to compare proportions was used to find statistical associations between clinical manifestations and each identified etiology. We used the Fisher’s exact test to compare proportions for variables with a value <5 in any of the boxes of the contingency table. A probability (P) value <0.05 was considered as statistically significant. Analyses of all variables were performed with the STATA 17.0 statistical software (StataCorp LLC, College Station, Texas) for Windows.
RESULTS
Study population characteristics
A total of 116 subjects were recruited between September 14th and December 21st of 2021, 60 (51.7%) were recruited in the municipality of Apartadó and 56 (48.3%) were recruited in the municipality of Villeta. No deaths were reported in Apartadó municipality, and only one fatal case was reported in the municipality of Villeta.
In Apartadó, 32 (53.3%) patients were male, the mean age was 24.8 years (±18.7 SD), and 41 (68.3%) lived in an urban area (municipal seat of the municipality). Most of the recruited patients were children (31.7%) and young adults (25%). Contact with different domestic and wild animal species was reported, with rodents (50%), dogs (43.3%), and cats (41.7%) being the most common. All subjects reported contact with different arthropods (Table 1).
Table 1 - Demographic and epidemiological characteristics of recruited febrile patients in Apartadó and Villeta municipalities.

In Villeta, 30 (53.6%) were male, the mean age was 31.8 (±17.4), and 47 (83.9%) lived in urban areas. Most of the patients were young adults (37.5%), old adults (23.2%) and middle-aged adults (21.5%). Contact with different animal species was also reported, with dogs (48.2%) and cats (37.5%) being the most common. Similarly, all subjects reported contact with different arthropod species. More information is summarized in Table 1.
Etiology of febrile illness in Apartadó
A confirmed etiology was found in 36 (60%) subjects in Apartadó. The most frequent etiology was DENV infection detected in 15 (25%) subjects. The second most frequent etiology was leptospirosis detected in 10 (16.7%) subjects. Malaria due to Plasmodium vivax was identified in 6 (10%) subjects. SARS-CoV-2 infection was detected in 5 (8.3%) subjects. SFG rickettsiosis in 4 (6.7%) subjects, and finally, CHIKV infection was detected in 1 (1.7%) subject. Five subjects had co-infections, three of them had DENV/Leptospira co-infection, one had DENV/SFG Rickettsia co-infection, and one had SFG Rickettsia/P. vivax co-infection. No cases of MAYV, OROV, VEEV, or YFV were detected. More information regarding the identified etiologies and the diagnostic results obtained in the municipality of Apartadó can be observed in Tables 2 and 3.
Table 2 - Etiology of acute undifferentiated febrile illness in Apartadó, Antioquia department.

Table 3 - Diagnostic tests and positive results for each etiology of acute undifferentiated febrile illness identified in Apartadó, Antioquia department.

Etiology of febrile illness in Villeta
A confirmed etiology was found in 31 (55.4%) subjects in Villeta. The most frequent etiology was SFG rickettsiosis detected in 22 (39.3%) subjects. The second most frequent etiology was leptospirosis detected in 12 (21.4%) subjects. DENV was detected in 2 (3.6%) subjects, and finally, malaria due to P. vivax was identified in 1 (1.8%) subject. Co-infections were detected in six subjects, all of whom had SFG Rickettsia/Leptospira co-infections. More information regarding the identified etiologies and the diagnostic results obtained in the municipality of Villeta can be observed in Tables 4 and 5.
Table 4 - Etiology of acute undifferentiated febrile illness in Villeta, Cundinamarca department.

Table 5 - Diagnostic tests and positive results for each etiology of acute undifferentiated febrile illness identified in Villeta, Cundinamarca department.

Temporal distribution of cases
A total of 15, 28, 27 and 46 patients were enrolled during September, October, November and December, respectively, in both sampling regions.
Considering patient recruitment by sampling area, in Apartadó, the largest number of patients were recruited in October (21 patients). DENV was the most frequent etiology identified in the region of our study during September (n=5) and October (n=7). During the last month, in most of the recruited patients no etiology was identified. And during November, leptospirosis, COVID-19 and SFG rickettsiosis (n=3) were the most frequent identified etiologies.
In the municipality of Villeta, most of the patients (n=33) were recruited in December, the remaining 23 febrile patients were recruited in the other three months of the study. During September (n=2), October (n=3), and mainly December (n=15), SFG rickettsiosis was the most frequently identified etiology. In November, leptospirosis (n=3) was identified as the most frequent cause of AUFI. More information regarding the distribution of febrile patients and each of the identified etiologies by months for each sampling site can be seen in Figure 2.
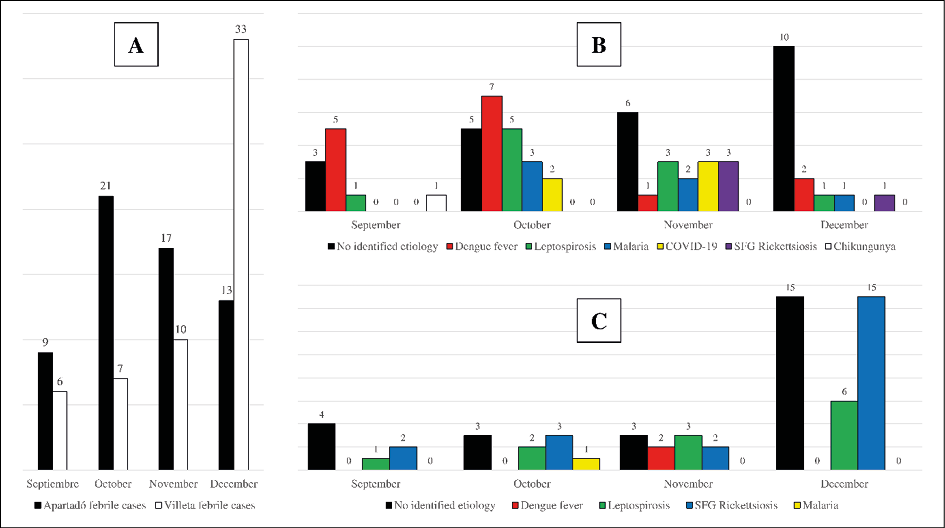
Figure 2 - Recruited patients and identified etiologies in Apartadó and Villeta municipalities during the course of the study. A) Number of febrile patients recruited in both municipalities per month. B) Identified etiologies per month in the municipality of Apartadó. C) Identified etiologies per month in the municipality of Villeta.
Clinical signs and symptoms
In addition to fever, six symptoms were present in more than 50% of the participants, including malaise in 105 (90.5%), headache in 93 (80.2%), chills in 75 (64.7%), sweats in 66 (56.9%), nausea in 66 (56.9%), and anorexia in 61 (52.6%). Common signs and symptoms among subjects with only one identified pathogen are compared in Table 6. All subjects with leptospirosis or COVID-19 had malaise. All subjects with malaria or COVID-19 had headache. All subjects with malaria also had chills and fever.
We found that chills (P=0.022), arthralgia (P=0.012) and nasal congestion (P=0.014) were significantly more frequent among subjects with SFG rickettsiosis. Coluria (P=0.036) was significantly more frequent in dengue fever cases than among those negative for DENV. Sweats (P=0.035) were more common in malaria cases than in non-malarial cases. No statistically significant differences were found for clinical manifestations present in patients with and without leptospirosis and COVID-19. Only one subject had CHIKV infection precluding any comparison of symptoms and statistical analysis.
Table 6 - Clinical characteristics of recruited febrile patients.

DISCUSSION
Six etiologies of AUFI were detected among febrile patients seeking medical attention in two regions of Colombia between September and December of 2021. In the Apartadó municipality, dengue fever (25%) and leptospirosis (16.7%) were the two most prominent causes of AUFI identified. A similar study performed over 10 years ago in three municipalities including Apartadó showed similar results with DENV as the most common cause of AUFI (37.3%), followed by leptospirosis (14.1%) and SFG rickettsiosis (2.7%) [24]. In Villeta municipality, SFG rickettsiosis (39.3%) and leptospirosis (21.4%) were identified as the two main causes of AUFI. A similar study performed six years ago in the same municipality identified leptospirosis (24%) as the main cause of AUFI, followed by dengue fever (16.4%) and SFG rickettsiosis (2.9%) [25]. We did observe geographical variation during our study. While in the municipality of Apartadó the main cause of AUFI identified was dengue fever, in the municipality of Villeta the main cause of AUFI was SFG rickettsiosis. No cases of MAYV, OROV, YFV or VEEV infections were detected by the multiplex PCR tests performed in our study. However, exposure to MAYV and OROV [36-38] and confirmed OROV infections [39] have been detected in Colombia. Transient viremia by these agents might have been missed and further testing using serology of paired acute and convalescent serum samples may help identify subjects with these infections. Furthermore, an association of clinical variables with the different etiologies detected in both regions was carried out, finding that some signs and symptoms were more frequent for some etiologies, and that some of these clinical variables showed statistically significant differences; however due to the small number of samples, it is possible that the statistical tests may not reflect the true picture even though the data for both municipalities were compiled together for the present study.
Dengue fever remains as the most important cause of AUFI in the Apartadó area due to high endemicity of the disease [40]. Cases of dengue fever with warning signs and severe dengue are diagnosed predominantly among young adults and children in Apartadó with a high mortality risk in the latter [41]. Studies performed in the region have identified several Aedes spp. which are potential vectors of DENV and other arboviruses [42]. Although, mosquito control measures have been implemented to prevent transmission in the region [43], dengue fever continues to be a significant problem in Apartadó as shown by our results.
The second most important cause of AUFI in Apartadó and Villeta municipalities was leptospirosis. This zoonotic infectious disease is considered an important public health problem in several municipalities of Colombia where fatal cases of the disease have been reported [27]. Transmission of leptospirosis in northern Colombia has been associated with contact with infected animals and environmental conditions [44]. Domestic and wild animals may play an important role in the eco-epidemiology of leptospirosis in these regions [45, 46]. Further studies of the animal hosts and factors affecting the transmission of leptospirosis in rural areas of Colombia, such as Apartadó and Villeta municipalities, may help to create prevention measures and limit human contact with infected animals or their excreta.
Malaria is considered one of the main public health problems in Colombia as more than 90% of the country’s territory is highly susceptible to malaria transmission [47]. In the present study, malaria was a frequent cause of AUFI in the municipality of Apartadó. The Urabá gulf region together with the Bajo Cauca and Alto Sinú regions are recognized as the most malaria endemic areas in Colombia. Most cases of malaria in the country are reported from these three regions and are mainly caused by P. vivax. Children under 5-years-old and pregnant women are more vulnerable to complicated and fatal forms of the disease [48, 49]. Transmission of malaria in Colombia is associated with anthropophilic anopheline species such as Anopheles nuneztovari, Anopheles darlingi and Anopheles albimanus that have a wide distribution in endemic areas. Other Anopheles species such as Anopheles triannulatus may be also involved in malaria transmission cycle in the country [50]. Control measures have been effective to decrease malaria incidence by as much as 72% in some areas. However, problems related to Plasmodium resistance to antimalaria medications, urbanization of the disease transmission, and the presence submicroscopic asymptomatic infected carriers have hindered malaria eradication efforts [51]. Thus, despite progress in control, malaria must be considered in the evaluation of AUFI in Colombia as shown in our study. Malaria cases have not been identified in the Villeta municipality before. The case diagnosed in our study represents the first detection of malaria due to P. vivax in this municipality. It has not been possible to confirm if this case was acquired through autochthonous transmission or was imported because the patient was lost to follow up. P. vivax can produce asymptomatic or clinically silent infections in endemic regions and these patients may present with AUFI caused by a different pathogen [52, 53]. Considering that Villeta municipality is not endemic for malaria this possibility is less likely.
SARS-CoV-2 as the only cause of AUFI was detected in the municipality of Apartadó. After the pandemic spread of SARS-CoV-2, COVID-19 has become an endemic infection worldwide [54]. Although diffuse pneumonia is the most severe presentation of SARS-CoV-2 [55], studies performed around the world have shown that COVID-19 may have protean clinical presentations. Atypical forms of COVID-19 may include AUFI with no evidence of respiratory symptoms which represents a great challenge in tropical areas where other etiologies are circulating [18, 56]. Although, COVID-19 must be included in the differential diagnosis of AUFI, it may not be a prominent cause depending on the specific transmission conditions (e.g., season of the year, vaccination levels) in some regions and misdiagnosis can occur [57].
SFG rickettsiosis was the most common cause of AUFI in Villeta municipality, and it was also identified as a cause of fever in Apartadó municipality. A previous study [25] described the presence of SFG rickettsiosis among subjects presenting with AUFI between October and March in the municipality of Villeta. However, contrasting with our study, SFG rickettsiosis was only the third most common cause of AUFI after leptospirosis and dengue fever [25]. Differences in inclusion criteria and season of subject inclusion may explain these variations in incidence between studies. Nonetheless, Villeta is recognized as one of the most important regions for SFG rickettsiosis due to R. rickettsii in Colombia. The first cases of SFG rickettsiosis due to R. rickettsii were detected in 1935 by Dr. Luis Patiño Camargo in the town of Tobia, in the vicinity of Villeta municipality, with a fatality rate of 95% [58]. Two other compatible fatal cases were later described in nearby areas [59]. After an epidemiological silence of more than 65 years, new fatal cases of SFG rickettsiosis and a high seropositivity against SFG rickettsiae among febrile and healthy population were reported in Villeta municipality [28, 60]. Eco-epidemiological studies have identified that cases of SFG rickettsiosis due to R. rickettsii are probably linked to Amblyomma patinoi ticks as vectors of the disease in the region [61]. Domestic and farm animals such as dogs and horses are also involved in the transmission cycle of this pathogen [62]. Considering the high fatality rate of SFG rickettsiosis reported in Villeta municipality, febrile cases in these areas must be carefully managed due to the high endemicity of R. rickettsii. Other less pathogenic Rickettsia spp. such as Rickettsia amblyommatis or Rickettsia parkeri may also be present as milder cases of SFG rickettsiosis without fatal outcomes and/or severe presentations in Villeta municipality [25]. Only one fatal case was described in our study population. This was a male subject that had positive DENV IgG antibodies and a titer of 1:64 for SFG rickettsiosis in the acute phase sample. No convalescent sample was obtained from this subject. In the Urabá gulf, human circulation of SFG Rickettsia spp. has been demonstrated by seropositivity rates and detection of incident cases in different municipalities of Northern Colombia near Apartadó municipality [63-65]. Circulation of Rickettsia spp. has also been described in rodents and ectoparasites [65], and two pathogenic species, R. rickettsii and R. parkeri, have been associated with human cases in the same regions [66, 67]. All the above highlight the importance of SFG rickettsiosis as a cause of AUFI in Colombia.
Although in the present study a large number of diagnostic methods have been implemented to determine the etiology of AUFI, all these tests have some limitations. Detection of NS1 and anti-DENV IgM antibodies had a low sensitivity in secondary infections and during the later stages of the disease; furthermore, IgM antibodies are not always detectable in the earliest stages of the disease [68]. Anti-DENV IgG antibodies can persist for several years, thus their results must be interpreted carefully, mainly in secondary infections [68]. Diagnosis of leptospirosis is highly complex since a single method is not sufficient to guarantee a definitive diagnosis [69]. Detection of anti-Leptospira IgM antibodies should be interpreted carefully; although they appear in the early stages of the disease and have a sensitivity of 84% during the acute phase of infection, they can persist for several months raising the question whether they identify a current infection [70]. MAT remains as the gold standard method, however, the sensitivity of this methods depends on the serovars evaluated, making it necessary to include local native strains to improve its effectiveness [69, 70]. Regarding malaria, diagnosis using thick blood smear and RDTs also has also some limitations, which have a low sensitivity in those infections where there is a low parasite density [71]. A four-fold seroconversion of anti-Rickettsia antibodies is not always evident, and depends on the time when the convalescent phase sample is obtained, requiring at least two or three weeks for the antibodies to be synthetized and thus be detected [72]. Molecular methods, such as PCR, have become very useful diagnostic tools due to their high sensitivity mainly in the early stages of the disease; however, they also have some limitations, such as false-negative results due to a low sample volume with a low concentration of microorganisms, and false-positive results due to background DNA contamination from previous PCR reactions [73].
The present study is part of larger multisite, multi-country study of AUFI carried on in six regions from four different countries: Apartadó and Villeta, Colombia; La Romana, Dominican Republic; Merida and Molas, Mexico; and Quillabamba, Peru. Although a common protocol backbone and data collection system were used in the studies conducted in these regions, the research teams and sampling time in each region are different. Furthermore, some additional protocols may have been incorporated and others avoided, making each of studies completely individualized from the others. The present study reports what was found in both regions from Colombia between September and December 2021.
CONCLUSIONS
The present study confirms the relevance of dengue fever, leptospirosis, SFG rickettsiosis, and malaria as causes of AUFI in the municipality of Apartadó, as well as the association of COVID-19 with undifferentiated febrile cases in the tropics. The great importance of SFG rickettsiosis as the main cause of fever in the municipality of Villeta in which a great number of cases may be occurring and are probably being misdiagnosed is of note. The potential detection of malaria due to P. vivax in Villeta warrants further studies and increased surveillance. Emerging and re-emerging arboviruses such as MAYV, OROV, VEEV and YFV were not detected in both municipalities.
Declaration of competing interest
The authors have no conflicts of interest to disclose in relation to this work.
Acknowledgements
We would like to recognize the collaboration of health authorities at the local institutions in Apartadó (Antonio Roldán Betancur Hospital) and Villeta (Salazar Hospital), in Antioquia and Cundinamarca departments (Colombia), respectively, and all the subjects that agreed to participate in the study. We also thank GIDRN – Global Infectious Disease Research Network and Minciencias for the financial support of the present study.
Members of the GIDRN – Global Infectious Diseases Research Network that are included in the present study as authors are Miguel M. Cabada, Patricia V. Aguilar, Juan David Rodas, Marylin Hidalgo, Francisco J. Diaz, Margarita Arboleda and Peter C. Melby. Other members of the GIDRN – Global Infectious Diseases Research Network are Karen Mozo, Eugenia S. Gonzalez-Diaz, Matilde Jimenez-Coello, Mathew M. Dacso, Antonio Ortega-Pacheco, David H. Walker, Scott C. Weaver and Robert Paulino-Ramirez.
Data availability statement
The data generated and analyzed during the current study are not publicly available, but they can be shared by request to the corresponding author.
Funding
This study was supported as part of the GIDRN – Global Infectious Disease Research Network, with funding from the Center for Tropical Diseases (CTD) and Division of Infectious Diseases at the University of Texas Medical Branch (UTMB); and by Minciencias, through grant # 111584467514, Project name: “Caracterización etiológica del sindrome febril agudo indiferenciado (SFAI) en dos regiones de Colombia”.
REFERENCES
- Mittal G, Ahmad S, Agarwal RK, et al. Aetiologies of acute undifferentiated febrile illness in adult patients - an experience from a tertiary care hospital in northern India. J Clin Diagn Res. 2015; 9 (12): DC22-4. doi: 10.7860/JCDR/2015/11168.6990.
- Thompson CN, Blacksell SD, Paris DH, et al. Undifferentiated febrile illness in Kathmandu, Nepal. Am J Trop Med Hyg. 2015; 92 (4): 875-878. doi: 10.4269/ajtmh.14-0709.
- Espinosa N, Cañas E, Bernabeu-Wittel M, et al. The changing etiology of fever of intermediate duration. Enferm Infecc Microbiol Clin. 2010; 28 (7): 416-420. doi: 10.1016/j.eimc.2009.07.014
- Oteo JA. Fiebre de duración intermedia: nuevos tiempos, nuevas herramientas y cambio de espectro. Enferm Infecc Microbiol Clin. 2010; 28 (7): 407-408. doi: 10.1016/j.eimc.2010.04.003.
- Cabrera R, Mendoza W, López-Mosquera L, et al. Tick-Borne-Agents detection in patients with acute febrile syndrome and ticks from Magdalena Medio, Colombia. Pathogens. 2022; 11 (10): 1090. doi: 10.3390/pathogens11101090.
- Jung HC, Chon SB, Oh WS, et al. Etiologies of acute undifferentiated fever and clinical prediction of scrub typhus in a non-tropical endemic area. Am J Trop Med Hyg. 2015; 92 (2): 256-261. doi: 10.4269/ajtmh.14-0377.
- Kamalia MA, Ayoub ME, Bureau BL, et al. Acute Fever of Unknown Origin: A Presentation of West Nile Encephalitis. WMJ. 2022; 121 (2): E22-E26.
- Mattar S, Tique V, Miranda J, et al. Undifferentiated tropical febrile illness in Cordoba, Colombia: Not everything is dengue. J Infect Public Health. 2017; 10 (5): 507-512. doi: 10.1016/j.jiph.2016.09.014.
- Moreira J, Bressan CS, Brasil P, et al. Epidemiology of acute febrile illness in Latin America. Clin Microbiol Infect. 2018; 24 (8): 827-835. doi: 10.1016/j.cmi.2018.05.001.
- Singhi S, Chaudhary D, Varghese GM, et al. Tropical fevers: Management guidelines. Indian J Crit Care Med. 2014; 18: 62-69. doi: 10.4103/0972-5229.126074.
- Bottieau E, Yansouni CP. Fever in the tropics: the ultimate clinical challenge? Clin Microbiol Infect. 2018; 24 (8): 806-807. doi: 10.1016/j.cmi.2018.06.018.
- Tun ZM, Moorthy M, Linster M, et al. Characteristics of acute febrile illness and determinants of illness recovery among adults presenting to Singapore primary care clinics. BMC Infect Dis. 2016; 16 (1): 612. doi: 10.1186/s12879-016-1958-4.
- Cortés JA, Romero-Moreno LF, Aguirre-León CA, et al. Enfoque clínico del síndrome febril agudo en Colombia. Infectio. 2017; 21 (1): 39-50.
- Ferreira MU, Castro MC. Malaria Situation in Latin America, and the Caribbean: Residual and Resurgent Transmission and Challenges for Control and Elimination. Methods Mol Biol. 2019; 2013: 57-70. doi: 10.1007/978-1-4939-9550-9_4.
- Santos LLM, de Aquino EC, Fernandes SM, et al. Dengue, chikungunya, and Zika virus infections in Latin America and the Caribbean: a systematic review. Rev Panam Salud Publica. 2023; 47: e34. doi: 10.26633/RPSP.2023.34.
- Moreira J, Barros J, Lapouble O, et al. When fever is not malaria in Latin America: a systematic review. BMC Med. 2020; 18 (1): 294. doi: 10.1186/s12916-020-01746-z.
- Gupta A, Siddiqui F, Purwar S, et al. Is it always COVID-19 in acute febrile illness in the tropics during the pandemic? PLoS Negl Trop Dis. 2022; 16 (11): e0010891. doi: 10.1371/journal.pntd.0010891.
- Nunthavichitra S, Prapaso S, Luvira V, et al. Case report: COVID-19 presenting as acute undifferentiated febrile illness-a tropical world threat. Am J Trop Med Hyg. 2020; 103 (1): 83-85. doi: 10.4269/ajtmh.20-0440.
- Silva-Ramos CR, Faccini-Martínez ÁA, Serna-Rivera CC, et al. Etiologies of zoonotic tropical febrile illnesses that are not part of the notifiable diseases in Colombia. Microorganisms. 2023; 11 (9): 2154. doi: 10.3390/microorganisms11092154.
- Nii-Trebi NI. Emerging and neglected infectious diseases: insights, advances, and challenges. Biomed Res Int. 2017; 2017: 5245021. doi: 10.1155/2017/5245021.
- Rangel-Churio JO. La biodiversidad de Colombia: significado y distribución regional. Revista Acad Colomb Ci Exact. 2015; 39 (51): 176-200.
- Pabón JD. El cambio climático global y su manifestación en Colombia. Cuad Geogr Rev Colomb Geogr. 2003; 12: 111-119.
- Villegas Vélez ÁA, Castrillón Gallego C. Territorio, enfermedad y población en la producción de la geografía tropical colombiana, 1872-1934. Hist Crit. 2006; 32: 94-117.
- Arroyave E, Londoño AF, Quintero JC, et al. Etiología y caracterización epidemiológica del síndrome febril no palúdico en tres municipios del Urabá antioqueño, Colombia. Biomedica. 2013; 33 (1): 99-107.
- Faccini-Martínez ÁA, Ramírez-Hernández A, Barreto C, et al. Epidemiology of spotted fever group rickettsioses and acute undifferentiated febrile illness in Villeta, Colombia. Am J Trop Med Hyg. 2017; 97 (3): 782-788. doi: 10.4269/ajtmh.16-0442.
- García-Henao JP, Alzate-Piedrahita JA, Guevara-Betancurt MP, et al. Acute Febrile Syndrome in an endemic region of Colombia: What is there beyond Dengue? Iatreia. 2021.
- Agudelo-Flórez P, Restrepo-Jaramillo BN, Arboleda-Naranjo M. Situación de la leptospirosis en el Urabá antioqueño colombiano: estudio seroepidemiológico y factores de riesgo en población general urbana. Cad Saude Publica. 2007; 23 (9): 2094-2102. doi: 10.1590/s0102-311x2007000900017.
- Hidalgo M, Sánchez R, Orejuela L, et al. Prevalence of antibodies against spotted fever group rickettsiae in a rural area of Colombia. Am J Trop Med Hyg. 2007; 77 (2): 378-380.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42 (2): 377-381. doi: 10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019; 95: 103208. doi: 10.1016/j.jbi.2019.103208.
- Singh B, Bobogare A, Cox-Singh J, et al. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999; 60 (4): 687-692. doi: 10.4269/ajtmh.1999.60.687.
- Rojas A, Stittleburg V, Cardozo F, et al. Real-time RT-PCR for the detection and quantitation of Oropouche virus. Diagn Microbiol Infect Dis. 2020; 96 (1): 114894. doi: 10.1016/j.diagmicrobio.2019.114894.
- Wu W, Wang J, Yu N, et al. Development of multiplex real-time reverse-transcriptase polymerase chain reaction assay for simultaneous detection of Zika, dengue, yellow fever, and chikungunya viruses in a single tube. J Med Virol. 2018; 90 (11): 1681-1686. doi: 10.1002/jmv.25253.
- Peláez Sánchez RG, Quintero JÁL, Pereira MM, et al. High-Resolution melting curve analysis of the 16S ribosomal gene to detect and identify pathogenic and saprophytic leptospira species in Colombian isolates. Am J Trop Med Hyg. 2017; 96 (5): 1031-1038. doi: 10.4269/ajtmh.16-0312.
- Blanton LS. Rickettsiales: Laboratory Diagnosis. In Sunil Thomas (Editor), Rickettsiales - biology, molecular biology, epidemiology, and vaccine development, Part II, Springer International Publishing. 2016: 95-108.
- Gil-Mora J, Acevedo-Gutiérrez LY, Betancourt-Ruiz PL, et al. Arbovirus antibody seroprevalence in the human population from Cauca, Colombia. Am J Trop Med Hyg. 2022; 107 (6): 1218-1225. doi: 10.4269/ajtmh.22-0120.
- Groot H. Estudios sobre virus transmitidos por artrópodos en Colombia. Rev Acad Colomb Cien Exac Fis Nat. 1964; 12: 3-23.
- Hidalgo M, Castañeda E, Méndez J, et al. Detección de anticuerpos contra arbovirus y rickettsias en sueros provenientes del programa centinela de entidades febriles, 2000-2004. Inf Quinc Epidemiol Nac. 2007; 12 (6): 81-96.
- Gómez-Camargo DE, Egurrola-Pedraza JA, Cruz CD, et al. Evidence of Oropouche Orthobunyavirus Infection, Colombia, 2017. Emerg Infect Dis. 2021; 27 (6): 1756-1758. doi: 10.3201/eid2706.204405.
- Restrepo B, Lopera T. Estudio seroepidemiologíco de dengue en la región del Urabá Antioqueño-Colombia. Infectio. 2004; 8 (4).
- Arboleda M, Campuzano M, Restrepo BN, et al. Caracterización clínica de los casos de dengue hospitalizados en la E.S.E. Hospital “Antonio Roldán Betancur”, Apartadó, Antioquia, Colombia, 2000. Biomedica. 2006; 26 (2): 286-294.
- Parra-Henao G, Suárez L. Mosquitos (Díptera: Culicidae) vectores potenciales de arbovirus en la región de Urabá, noroccidente de Colombia. Biomedica. 2012; 32(2): 252-262. doi: 10.1590/S0120-41572012000300013.
- Alarcón ÉP, Segura ÁM, Rúa-Uribe G, et al. Evaluación de ovitrampas para vigilancia y control de Aedes aegypti en dos centros urbanos del Urabá antioqueño. Biomedica. 2014; 34 (3): 409-424. doi: 10.1590/S0120-41572014000300011.
- Quintero-Vélez JC, Rodas JD, Rojas CA, et al. Leptospira infection in rural areas of Urabá Region, Colombia: a prospective study. Am J Trop Med Hyg. 2022; 107 (6): 1267-1277. doi: 10.4269/ajtmh.21-1103.
- Monroy FP, Solari S, Lopez JÁ, et al. High Diversity of Leptospira Species Infecting Bats Captured in the Urabá Region (Antioquia-Colombia). Microorganisms. 2021; 9 (9): 1897. doi: 10.3390/microorganisms9091897.
- Perez-Garcia J, Monroy FP, Agudelo-Florez P. Canine Leptospirosis in a northwestern region of Colombia: serological, molecular and epidemiological factors. Pathogens. 2022; 11 (9): 1040. doi: 10.3390/pathogens11091040.
- Padilla-Rodríguez JC, Olivera MJ, Ahumada-Franco ML, et al. Malaria risk stratification in Colombia 2010 to 2019. PLoS One. 2021; 16 (3): e0247811. doi: 10.1371/journal.pone.0247811.
- Arboleda M, Pérez MF, Fernández D, et al. Perfil clínico y de laboratorio de los pacientes con malaria por Plasmodium vivax, hospitalizados en Apartadó, Colombia. Biomedica. 2012; 32 (1): 58-67. doi: 10.1590/S0120-41572012000500008.
- Vásquez AM, Zuluaga-Idárraga L, Arboleda M, et al. Malaria in pregnancy in endemic regions of Colombia: high frequency of asymptomatic and peri-urban infections in pregnant women with malaria. Infect Dis Obstet Gynecol. 2020; 2020: 2750258. doi: 10.1155/2020/2750258.
- Naranjo-Diaz N, Rosero DA, Rua-Uribe G, et al. Abundance, behavior and entomological inoculation rates of anthropophilic anophelines from a primary Colombian malaria endemic area. Parasit Vectors. 2013; 6: 61. doi: 10.1186/1756-3305-6-61.
- Recht J, Siqueira AM, Monteiro WM, et al. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J. 2017; 16 (1): 273. doi: 10.1186/s12936-017-1925-6.
- Barros LB, Calil PR, Rodrigues PT, et al. Clinically silent Plasmodium vivax infections in native Amazonians of northwestern Brazil: acquired immunity or low parasite virulence? Mem Inst Oswaldo Cruz. 2022; 117: e220175. doi: 10.1590/0074-02760220175.
- Martin TCS, Vinetz JM. Asymptomatic Plasmodium vivax parasitaemia in the low-transmission setting: the role for a population-based transmission-blocking vaccine for malaria elimination. Malar J. 2018; 17 (1): 89. doi: 10.1186/s12936-018-2243-3.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382 (8): 727-733. doi: 10.1056/NEJMoa2001017.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395 (10223): 497-506. doi: 10.1016/S0140-6736(20)30183-5.
- Yang Z, Chen X, Huang R, et al. Atypical presentations of coronavirus disease 2019 (COVID-19) from onset to readmission. BMC Infect Dis. 2021; 21 (1): 127. doi: 10.1186/s12879-020-05751-8.
- Silva-Ramos CR, Mejorano-Fonseca JA, Rodríguez-Morales AJ, et al. Zoonotic febrile illnesses misdiagnosed as COVID-19: a review of reported clinical cases. Infez Med. 2023; 31 (2): 151-162. doi: 10.53854/liim-3102-3.
- Patiño L, Afanador A, Paul JH. A Spotted Fever in Tobia, Colombia Preliminary Report. Am J Trop Med Hyg. 1937; 17: 639-53.
- Patiño-Camargo L. Nuevas observaciones sobre un tercer foco de fiebre petequial (maculosa) en el hemisferio americano. Bol Ofic Sanit Panamericana. 1941; 20: 1112-1124.
- Hidalgo M, Orejuela L, Fuya P, et al. Rocky Mountain spotted fever, Colombia. Emerg Infect Dis. 2007; 13 (7): 1058-1060. doi: 10.3201/eid1307.060537.
- Faccini-Martínez ÁA, Costa FB, Hayama-Ueno TE, et al. Rickettsia rickettsii in Amblyomma patinoi ticks, Colombia. Emerg Infect Dis. 2015; 21 (3): 537-539. doi: 10.3201/eid2013.140721.
- Hidalgo M, Vesga JF, Lizarazo D, et al. A survey of antibodies against Rickettsia rickettsii and Ehrlichia chafeensis in domestic animals from a rural area of Colombia. Am J Trop Med Hyg. 2009; 80 (6): 1029-1030.
- Quintero Vélez JC, Aguirre-Acevedo DC, Rodas JD, et al. Epidemiological characterization of incident cases of Rickettsia infection in rural areas of Urabá region, Colombia. PLoS Negl Trop Dis. 2018; 12 (10): e0006911. doi: 10.1371/journal.pntd.0006911.
- Gil-Lora EJ, Patiño-Gallego JJ, Acevedo-Gutiérrez LY, et al. Infección y enfermedad por Rickettsia spp. del grupo de las fiebres manchadas en pacientes febriles del Urabá antioqueño, Colombia. Iatreia. 2019; 32 (3): 167-176. https://doi.org/10.17533/udea.iatreia.15.
- Quintero JC, Londoño AF, Díaz FJ, et al. Ecoepidemiología de la infección por rickettsias en roedores, ectoparásitos y humanos en el noroeste de Antioquia, Colombia. Biomédica. 2013; 33 (1): 38-51. https://doi.org/10.7705/biomedica.v33i0.735.
- Arboleda M, Acevedo-Gutiérrez LY, Ávila A, et al. Human Rickettsiosis Caused by Rickettsia parkeri Strain Atlantic Rainforest, Urabá, Colombia. Emerg Infect Dis. 2020; 26 (12): 3048-3050. doi: 10.3201/eid2612.200388.
- Quintero Vélez JC, Faccini-Martínez ÁA, Rodas González JD, et al. Fatal Rickettsia rickettsii infection in a child, Northwestern Colombia, 2017. Ticks Tick Borne Dis. 2019; 10 (5): 995-996. doi: 10.1016/j.ttbdis.2019.05.009.
- Luvira V, Thawornkuno C, Lawpoolsri S, et al. Diagnostic performance of Dengue NS1 and antibodies by serum concentration technique. Trop Med Infect Dis. 2023; 8 (2): 117. doi: 10.3390/tropicalmed8020117.
- Pérez-García J, Agudelo-Flórez P, Parra-Henao GJ, et al. Incidencia y subregistro de casos de leptospirosis diagnosticados con tres métodos diferentes en Urabá, Colombia. Biomedica. 2019; 39 (s1): 150-162. doi: 10.7705/biomedica.v39i0.4577.
- Budihal SV, Perwez K. Leptospirosis diagnosis: competency of various laboratory tests. J Clin Diagn Res. 2014; 8 (1): 199-202. doi: 10.7860/JCDR/2014/6593.3950.
- Dahal P, Khanal B, Rai K, et al. Challenges in laboratory diagnosis of malaria in a low-resource country at tertiary care in Eastern Nepal: a comparative study of conventional vs. molecular methodologies. J Trop Med. 2021: 3811318. doi: 10.1155/2021/3811318.
- Stewart AG, Stewart AGA. An update on the laboratory diagnosis of Rickettsia spp. Infection. Pathogens. 2021; 10 (10): 1319. doi: 10.3390/pathogens10101319.
- Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004; 4 (6): 337-348. doi: 10.1016/S1473-3099(04)01044-8.