Le Infezioni in Medicina, n. 3, 403-411, 2022
doi: 10.53854/liim-3003-8
ORIGINAL ARTICLES
Comparison of clinical characteristics and outcome in RT-PCR positive and false-negative RT-PCR for COVID-19: A Retrospective analysis
Durga Shankar Meena1, Bharat Kumar1, Arjun Kachhwaha1, Deepak Kumar1, Satyendra Khichar1, Gopal Krishana Bohra1, Ankur Sharma2, Nikhil Kothari3, Pawan Garg4, Binit Sureka4, Mithu Banerjee5, Mahendra Kumar Garg1, Sanjeev Misra6
1Department of Internal Medicine, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India;
2Department of Trauma and Emergency (Anaesthesiology), All India Institute of Medical Sciences,Jodhpur, Rajasthan, India;
3Department of Anaesthesiology and Critical Care, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India;
4Department of Diagnostic and Interventional Radiology, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India;
5Department of Biochemistry, All India Institute of Medical Sciences, Jodhpur, Rajasthan, India;
6All India Institute of Medical Sciences, Jodhpur, Rajasthan, India
Article received 4 April 2022, accepted 8 June 2022
Corresponding author
Bharat Kumar
E-mail: drbharatmaheshwari@gmail.com
SummaRY
Cases with SARS-CoV-2 RT-PCR negative pneumonia are an understudied group with uncertainty remaining regarding their treatment approach. We aimed to compare the clinical and radiological characteristics of RT-PCR positive and clinically diagnosed RT-PCR negative COVID-19. This was a single-centre retrospective study conducted at a tertiary care hospital in Western India. All patients (age ≥18 years) with suspicion of COVID-19 with SARI (severe acute respiratory infections) who were subjected to RT-PCR testing (nasal/oropharyngeal swab) were included. Based on RT-PCR results, patients were categorized and compared for demographic, clinical, and biochemical characteristics and outcomes. Out of 500 patients, 339 (67.8%) found RT-PCR positive. Except for the radiological findings, both groups differ in clinical presentation, disease severity (inflammatory markers), and outcome. RT-PCR-positive patients had raised ferritin, NLR (Neutrophil-Lymphocyte ratio), LDH, and high mortality compared to the swab-negative group. In-hospital mortality was also significantly high in RT-PCR positive group (HR=1.9, 95% CI=1.4-2.5, p=0.001). On multivariate analysis, NLR, ferritin, and d-dimer were the independent predictors of mortality in RT-PCR-positive (p=0.038, 0.054, and 0.023). At the same time, raised TLC (total leukocyte count) and procalcitonin were the risk factors for poor outcomes in RT-PCR-negative patients (p=0.041 and 0.038). We found significantly raised ferritin, NLR, and LDH levels and increased mortality in RT-PCR positive patients compared to RT-PCR negative. Incorporating clinical features, radiological, and biochemical parameters could be prudent while managing the RT-PCR-negative patients.
Keywords: COVID-19, pneumonia, SARI, RT-PCR, CT-Thorax.
INTRODUCTION
The term ‘SARI’ (Severe Acute Respiratory Infection) was initially defined by the World Health Organization (WHO) in 2011 for global surveillance of respiratory infections. SARI is defined as an acute respiratory illness with a history of fever or measured temperature ≥38 C° and cough, onset within the last ten days, and requiring hospitalization [1]. During the current COVID-19 (Corona Virus Disease-2019) pandemic, there has been an overwhelming burden of SARI cases. Severe respiratory illness can be seen in nearly 14% of the patients with COVID-19, with a 2% mortality rate [2]. RT-PCR (real-time reverse transcription-polymerase chain reaction) assay is the only available method for the direct confirmation of COVID-19 [3]. Although RT-PCR has excellent specificity, its sensitivity remains questionable, resulting in false-negative reports [4, 5]. A recent metanalysis described the pooled false-negative RT-PCR results in 12% of the patients (ranges from 2% to 58%) [6]. However, there was insufficient certainty evidence due to the considerable heterogeneity of included studies.
False-negative results can occur due to improper collection of specimens, different timing of patient presentation, very low viral load, and laboratory errors [7, 8]. The majority of RT-PCR negative (false-negative RT-PCR for SARS-CoV-2) patients have radiological evidence (CT-Thorax) and clinical findings similar to RT-PCR positive patients; however, the clinical course and further management are uncertain. Some of the SARI cases may be attributed to pulmonary edema or other atypical viral infections on subsequent evaluation; however, many cases remain without alternate aetiology (false-negative RT-PCR), and the dilemma remains whether to treat them as RT-PCR positive. There are few reports which compared the COVID-19 patients based on RT-PCR results with conflicting observations [9-13]. Furthermore, studies on the Indian population are also lacking in this regard. This study aims to compare the clinical presentation, biochemical/radiological characteristics, and outcome of RT-PCR positive and negative COVID-19 patients.
PATIENTS AND METHODS
Study design, setting and participants
This was a retrospective observational study conducted at a tertiary care centre in Western India. All clinically suspected cases of COVID-19 who were admitted to the SARI ward were included. All cases of SARI presented between 1st April 2021 to 31st July 2021 were analysed after the approval of the institutional ethical committee (reference no - IMS/IEC/2021/3546).
Case definition and data collection
The definition of COVID-19 cases was adapted from guidelines from the Ministry of Health and Family Welfare (Government of India) [13]. SARI cases were defined as acute respiratory infection with a history of fever or measured fever of ≥38°C, and cough; with onset within the last 10 days; and requires hospitalization [13]. Clinical confirmed COVID-19 cases were defined as a person with a positive Nucleic Acid Amplification Test (NAAT), including RT-PCR or any other similar test approved by ICMR (Indian Council of Medical Research). The ‘TRUPCR SARS-Co V-2 Kit’ was the RT-PCR assay used in this study which was validated by the ICMR. Those who were RT-PCR negative but had clinical and radiological findings (X-ray chest or CT thorax) were considered RT-PCR negative clinical COVID-19 cases. Laboratory confirmation was done by obtaining a nasopharyngeal swab and performing RT-PCR assay to detect SARS-CoV-2. Patients who were found RT-PCR negative on the day of admission but had strong clinical suspicion of COVID-19 were subjected to a repeat swab test after 48 hours. Those who found RT-PCR positive in repeat test were excluded from the analysis. RTPCR negative patients were further evaluated for possible alternative aetiology (e.g., pulmonary oedema, other viral or atypical bacterial infections, exacerbation of interstitial lung disease, fungal infections). The investigations to rule out alternate aetiology were formulated at the discretion of the treating clinician with the help of a multidisciplinary team. Depending on the clinical presentation and underlying comorbidities, the echocardiography, cardiac biomarkers, CT-thorax, sputum analysis, bronchoalveolar lavage and autoimmune workup were performed to identify the alternate cause. After ruling out these causes, RTPCR negative patients were included for further analysis. Severe COVID-19 disease was defined as respiratory rate >30/min, breathlessness, or patients with Spo2 <90% [13].
We searched electronic hospital records for all patients admitted with SARI between April 2021 to July 2021. All demographic data (age, gender), clinical history, laboratory and radiologic characteristics, and outcome of each patient were extracted. Patients were divided into two groups based on RT-PCR positivity and compared. Clinical outcomes were assessed in terms of in-hospital mortality. In-hospital mortality rate was defined as the percentage of patients with COVID-19 who died in the hospital. We also searched the various predictors of mortality in each group.
CT score assessment
The CT severity score was calculated based on lung involvement (percentage) by scoring the percentage of each lobe involvement individually and given a score from 1 to 5 where Score 1: <5% involvement, Score 2: 5-25% involvement, Score 3: 26-50% involvement, Score 4: 51-75% involvement and, Score 5: >75% involvement. The final score will be the sum of individual lobar scores (out of 25 points).
Statistical analysis
Statistical analysis was performed using a statistical software package for social sciences (SPSS) version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp). Categorical data were expressed in percentages or frequencies and compared with the Chi-square test. Continuous variables were expressed in mean (Standard deviation) and Median (in cases with skewed data) and compared with independent students’ t-test and Mann-Whitney test. For the identification of predictors of mortality, univariate and multivariate logistic regression analyses were performed for different variables. In univariate analysis, variables with a p<0.05 were further analysed by multivariate analysis to find out independent association with outcome. Kaplan-Meier curve was performed to estimate survival probabilities, and the log-rank test was performed to analyse the significance of differences in survival curves between groups. The outcome of survival probabilities was reported on day ten after admission. As the final disposition of all patients was reported up to the last day of discharge or death (i.e. day 10), it was selected for this study.
RESULTS
A total of five hundred consecutive SARI patients were analysed in this study. Out of 500 patients, one hundred sixty-one patients were RT-PCR negative (32.2%). Patients who were found RT-PCR negative on the day of admission but had strong clinical suspicion of COVID-19 were subjected to a repeat swab test after 48 hours. However, we have excluded patients from the analysis who came out positive in subsequent testing (n=16). Demographic factors and clinical presentation of both groups are given in Table 1. RT-PCR positive patients were significantly older and hypertensive compared with RT-PCR negative patients, but the number of diabetics did not differ significantly in both groups (29.8% vs 24.2%, p=0.20). In contrast, RT-PCR-negative patients had a positive history of chronic obstructive pulmonary diseases and interstitial lung diseases (14.3% vs 3.5%, p<0.001). RT-PCR positive patients often presented with respiratory symptoms like fever, cough, and shortness of breath. In contrast, extra-pulmonary symptoms like diarrhoea, vomiting, headache, and myalgia were more common in RT-PCR negative patients (Table 1). More RT-PCR patients required oxygen by high flow nasal cannula on admission compared with RT-PCR negative patients (Table 1). In addition, the proportion of disease severity was more in the RT-PCR-positive group than in RT-PCR negative patients (80% vs 41%, p<0.001).
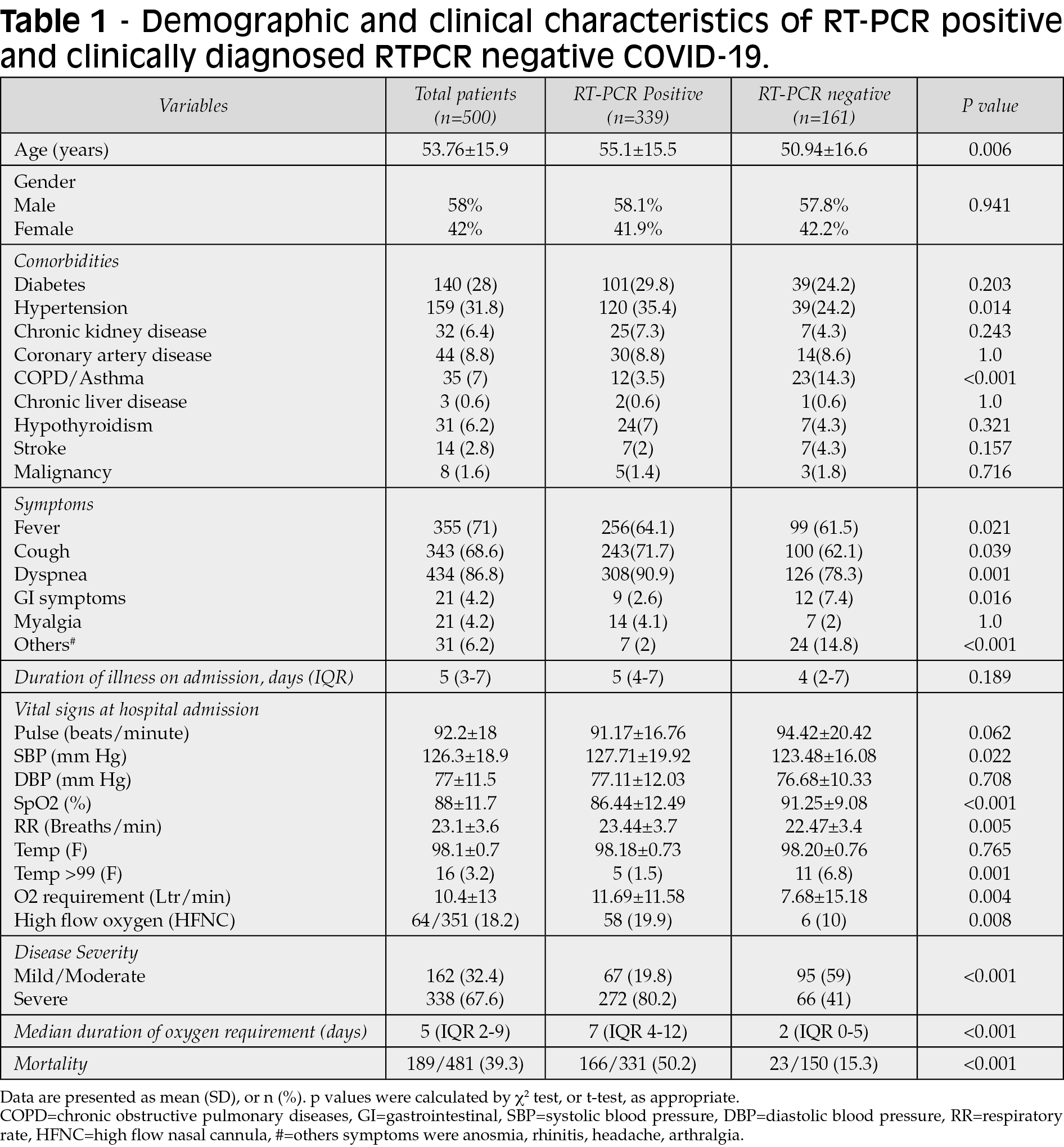
CT-thorax findings did not differ significantly between RT-PCR positive and negative patients (Table 2). The consolidation and pleural effusion incidence were similar in RT-PCR positive and negative groups (Table 2). The mean random plasma glucose (p=0.03), NLR (neutrophilic-lymphocytic ratio) (p=0.013), AST (aspartate transaminase) (p=0.001), ALT (alanine aminotransferase) (p=0.018), and BUN (blood urea nitrogen) (p=0.001) were significantly higher in RT-PCR-positive patients as compared with RT-PCR negative patients (Table 2). Among the various inflammatory markers, only serum ferritin and LDH (lactate dehydrogenase) levels were significantly increased in RT-PCR positive group compared to negative patients (p=0.001 and 0.012, respectively). Other inflammatory markers like HsCRP (High sensitivity C-reactive protein), procalcitonin, IL-6 (interleukin-6) and D-dimer did not differ between the two groups (Table-2).
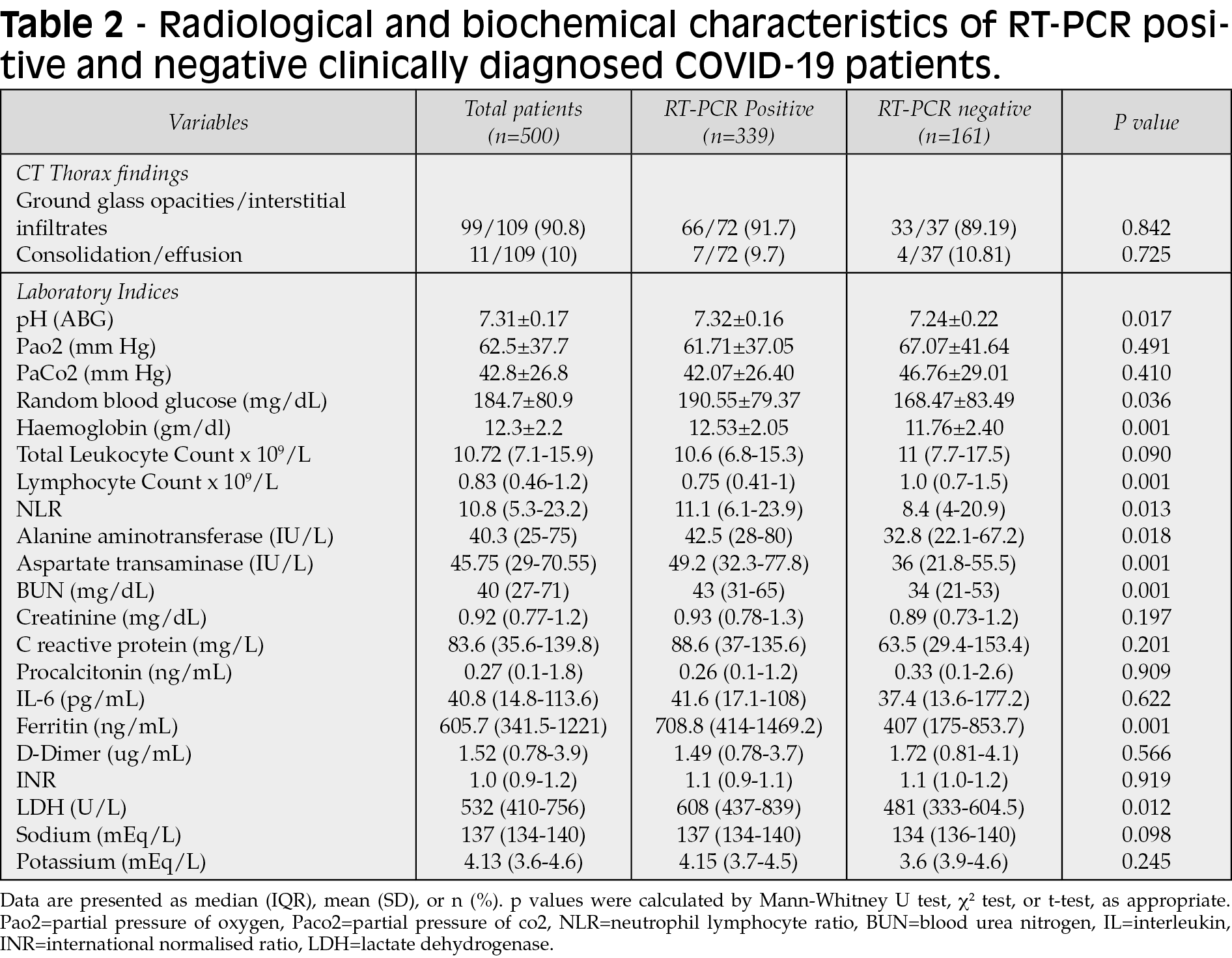
The median duration of oxygen requirement during hospitalization was also significantly higher in RT-PCR positive patients when compared with RT-PCR negative patients (7 days vs 2 days, p-value <0.001). Furthermore, the mortality rate was significantly higher in RT-PCR-positive patients than in RT-PCR negative patients (50.2% vs 15.3%, p-value <0.001). RT-PCR positive patients were more likely to have an adverse outcome (death) when compared to RT-PCR negative patients (The ten days hazard ratio =1.9, p=0.001, Figure 1).
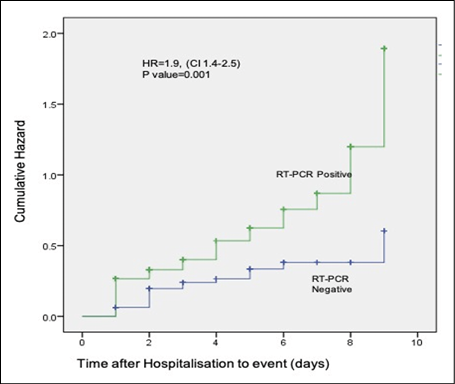
Figure 1 - Cox Regression analysis showing cumulative Hazard of death in COVID-19 patients based on RT-PCR positivity.
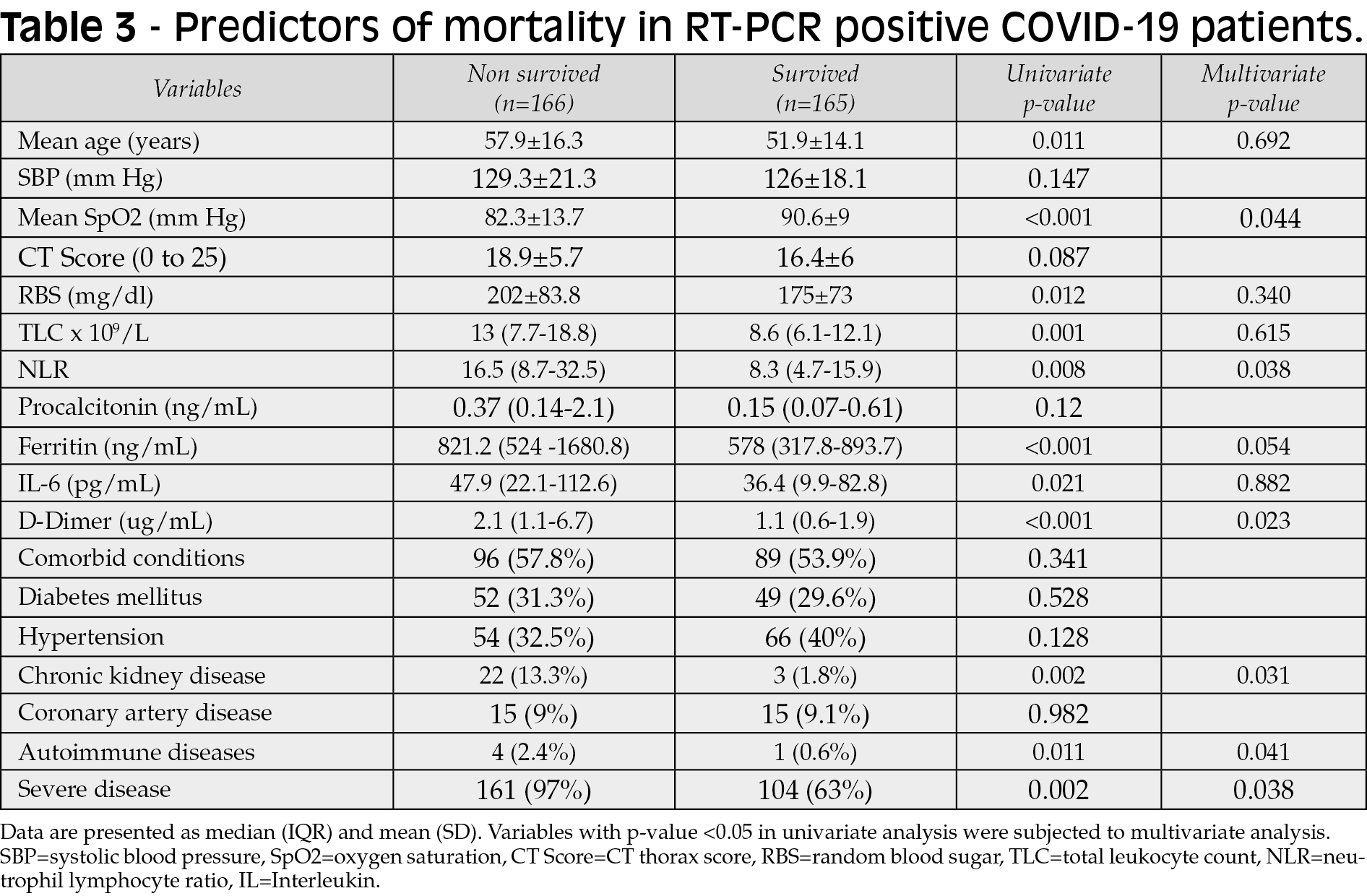
The various predictors of mortality in RT-PCR positive and negative patients are depicted in Table 3 and Table 4. RT-PCR positive patients who died were significantly older and had lower Spo2, high TLC (total leukocyte counts), high NLR, and high plasma glucose on admission compared to those who survived (Table 3). Similarly, high ferritin and D-dimer levels, chronic kidney disease (CKD), malignancy, and severe COVID-19 were also significantly associated with poor outcomes (Table 3). Multivariate analysis showed that lower Spo2, high NLR, high ferritin, high D-dimer, CKD, malignancy and severe COVID-19 were the independent factors associated with high mortality in RT-PCR-positive patients. A similar analysis for RT-PCR negative patients revealed only two lab variables (raised TLC and serum procalcitonin) associated with mortality in univariate and multivariate analysis (Table 4). RT-PCR negative patients who died had significant high TLC (16.4 x 109/L vs 10.3x109/L, p=0.04) and procalcitonin (1.8 vs 0.1 ng/ml, p=0.03). No significant difference was found for age, CT score, NLR, and other inflammatory markers (Table-4). In addition, hypertension, coronary artery diseases, and CKD were also found to be independent predictors of mortality in multivariate analysis (Table 4).

DISCUSSION
This study compared the clinical characteristics and outcomes of COVID-19 patients based on RT-PCR positivity. The RT-PCR assay is currently considered a gold standard for COVID-19 diagnosis [14]. However, there are various pitfalls while interpreting RT-PCR assay in COVID-19 patients. According to different studies, the sensitivity of RT-PCR assay varies from 70% to 85% [15-17]. False-negative reports can occur in human/laboratory errors, quality and type of specimen collected, the timing of the clinical course of the disease, a mutation in the viral genome, and mismatch between primer and probes [8,14,18]. This report found RT-PCR positivity in 67.8% of the SARI patients. This result was similar to previous studies, which reported RT-PCR positivity ranges from 59.2% to 85.8% [10, 11]. Di Paolo et al. discussed the possibility of positive RT-PCR in repeat testing, which was 4% in their report [19]. In our study, around 9% of patients (n=16) were found RT-PCR positive in repeat testing, which was relatively high compared to the aforementioned study.
The clinical presentation of RT-PCR positive patients was significantly different from RT-PCR negative patients. Pulmonary symptoms like fever, cough and shortness of breath were commonly found in RT-PCR-positive patients. At the same time, extrapulmonary features (e.g., diarrhoea, pain abdomen, and headache) were more commonly associated with the RT-PCR-negative group. In contrast, some reports described similar clinical presentations in both groups [11, 20, 21].
To establish the diagnosis, some reports advocate the use of CT-Thorax in RT-PCR negative patients, which can help in guiding the management [22, 23]. However, the literature showed conflicting evidence regarding this approach. Korkmaz et al. described similar CT-thorax findings (bilateral grand-glass opacities) in RT-PCR positive and negative patients and recommended the same treatment strategies in both groups [22]. Interestingly, our report showed that the incidence of consolidation and effusion in CT-thorax did not differ in RT-PCR negative and positive COVID-19. This result contradicts previous studies that described the increased incidence of consolidation and effusion in RT-PCR negative patients [23]. The association of CT consolidation findings with RT-PCR negativity probably reflects the non-COVID causes of pneumonia in the aforementioned reports.
In this study, we observed high NLR (11.1) in RT-PCR-positive patients. Previous reports have also shown both diagnostic and prognostic utility of NLR in COVID-19 patients [24-27]. Nalbant et al. described NLR as an independent predictor for the diagnosis of COVID-19 [24]. In their report, the risk of COVID-19 was 20.3 fold higher when NLR was >2.4 (p=0.007) [24]. Similarly, another report by Yang et al. described the increased risk of COVID-19 with high NLR (OR=2.4, p=0.019) [28]. There is some concern about the impact of corticosteroids on NLR [29]. Still, NLR is a readily available and cheap option, and a combination of NLR with CT findings can help diagnose COVID-19.
There was high mortality in RT-PCR positive patients despite similar CT-thorax findings when compared to RT-PCR negative group. This indicates the poor correlation of CT findings with the outcome. In contrast, high AST, LDH, and ferritin levels were observed in RT-PCR-positive patients, indicating high inflammation and poor prognosis. Similar observations were also described in previous reports [30, 31]. RT-PCR negative patients had less oxygen requirement (number of days on oxygen) and a better in-hospital survival rate. Middleton et al. described a 60% lower probability of death and duration of hospital stay in RT-PCR negative patients [10]. Interestingly, the median duration of illness till admission did not differ between RT-PCR positive and negative groups. This reduces the possibility of false-negative results based on the timing of specimen collection in our report. Contrary to that, previous reports showed a delayed presentation of swab-negative patients (7 days vs 6 days, p<0.001), which could have produced false-negative results [10]. In RT-PCR positive patients, ferritin, NLR, and D-dimer were the risk factors for mortality, reflecting the inflammatory cascade and coagulopathy. Notwithstanding, there could be several factors responsible for the difference in mortality in both groups. Although, the treatment given was similar in both groups. We speculate that the viral load, immune status, elderly population, and vaccine status are the various factors that could have affected the outcome. Another critical factor is the possibility of misclassification bias because not every patient underwent repeat RT-PCR testing, and the dilemma remains whether these patients were actual RT-PCR negative or not.
The clinical management of RT-PCR-negative COVID-19 patients is still a debated territory. Considering them false-negative will lead to unnecessary isolation and ethical issues and increase strain on health resources. At the same time, treating these patients as true swab-negative may increase the risk of disease spread, especially in healthcare settings. We emphasize the holistic approach, the patients with initial RT-PCR negative but raised NLR, LDH, and ferritin and positive CT findings should be subjected to repeated sampling. The utilization of serological assay (SARS-CoV-2 IgM/IgG) is another approach proposed by various reports [21]. Li et al. demonstrated the presence of SARS-CoV-2 IgM antibodies in 87% of the RT-PCR negative SARI patients [21]. If the appropriate time window is used (3-7 days from onset of symptoms for IgM and 10 days to 60 days for IgG), serological assay in conjunction with aforementioned inflammatory markers and CT thorax could be a guiding factor in the management of RT-PCR negative patients.
This study had a few limitations. Due to the study’s retrospective nature, it is possible to have some confounding factors. The serological assay was not performed, especially in RT-PCR negative patients. In addition, the impact of SARS-CoV2 variants on RT-PCR positivity was not studied in this report, which could be an important factor in false-negative RT-PCR results. Long-term pulmonary manifestations were not compared. Despite investigating alternate diagnoses, there is always uncertainty whether RT-PCR negative patients had COVID-19. Finally, this was a single-centre study, which may preclude its applicability in all RT-PCR negative populations.
In conclusion, this study highlighted the clinical spectrum of RT-PCR negative clinically diagnosed COVID-19 patients. Although the radiological presentation was similar to RT-PCR-positive, symptoms, severity, inflammatory markers, and clinical outcome differed. Whether these patients should be considered true RT-PCR negative or false negative is a subject of further research. RT-PCR negative patients had better outcomes suggesting either lower viral load or better immunity, contributing to RT-PCR negativity. Management of COVID-19 patients should not depend exclusively on RT-PCR positivity; clinicians should corroborate the clinical features and inflammatory and serological assay. Larger studies or metanalysis are needed to further explore the clinical characteristic of RT-PCR negative COVID-19 SARI patients.
Funding
None
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the institutional ethical committee of All India Institutes of Medical Sciences Jodhpur, Rajasthan (Reference No - AIIMS/IEC/2021/3546).
REFERENCES
[1] Fitzner J, Qasmieh S, Mounts AW, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018; 96, 122-8.
[2] Gupta N, Praharaj I, Bhatnagar T, et al; ICMR COVID Team. Severe acute respiratory illness surveillance for coronavirus disease 2019, India, 2020. Indian J Med Res. 2020; 151, 236-40.
[3] Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020; 55, 105955.
[4] Ferrari A, Alfano G, Guaraldi G. Persistent SARS-CoV-2 positivity: An intriguing puzzle among reinfection, RNA remnants and genomic integration in COVID-19. Infect Dis Clin Pract (Baltim Md). 2021; 29, e328-e329.
[5] Woloshin S, Patel N, Kesselheim AS. False Negative Tests for SARS-CoV-2 Infection - Challenges and Implications. N Engl J Med. 2020; 383, e38.
[6] Pecoraro V, Negro A, Pirotti T, Trenti T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur J Clin Invest. 2022; 52, e13706.
[7] Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: A systematic review. PLoS One. 2020; 15, e0242958.
[8] Kanji JN, Zelyas N, MacDonald C, et al. False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol J. 2021; 18: 13.
[9] Gaipov A, Gusmanov A, Abbay A, et al. Correction to: SARS-CoV-2 PCR-positive and PCR-negative cases of pneumonia admitted to the hospital during the peak of COVID-19 pandemic: analysis of in-hospital and posthospital mortality. BMC Infect Dis. 2021; 21, 692.
[10] Middleton P, Perez-Guzman PN, Cheng A, et al. Characteristics and outcomes of clinically diagnosed RT-PCR swab negative COVID-19: a retrospective cohort study. Sci Rep. 2021; 11, 2455.
[11] Zhang JJ, Cao YY, Dong X, et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2. Allergy. 2020; 75: 1809-12.
[12] Karimi F, Vaezi AA, Qorbani M, et al. Clinical and laboratory findings in COVID-19 adult hospitalized patients from Alborz province/Iran: comparison of rRT-PCR positive and negative. BMC Infect Dis. 2021; 21, 256.
[13] COVID-19 update, COVID-19 India Ministry of health and family Welfare. MoHFW. 2021. Available online at: https://www.mohfw.gov.in/ Accessed 01 June 2021.
[14] Goudouris ES. Laboratory diagnosis of COVID-19. J Pediatr (Rio J). 2021; 97, 7-12.
[15] Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. Brit Med J. 2020; 12,369:m1808.
[16] Kortela E, Kirjavainen V, Ahava MJ, et al. Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients. PLoS One. 2021; 16: e0251661.
[17] Clerici B, Muscatello A, Bai F, et al. Sensitivity of SARS-CoV-2 Detection With Nasopharyngeal Swabs. Front Public Health. 2021; 8, 593491.
[18] Hernández-Huerta MT Ph D, Pérez-Campos Mayoral L Ph D, Sánchez Navarro LM, et al. Should RT-PCR be considered a gold standard in the diagnosis of COVID-19? J Med Virol. 2021; 93, 137-8.
[19] Di Paolo M, Iacovelli A, Olmati F, et al. False-negative RT-PCR in SARS-CoV-2 disease: experience from an Italian COVID-19 unit. ERJ Open Res. 2020; 6, 00324-2020.
[20] Li YY, Wang WN, Lei Y, et al. Comparison of the clinical characteristics between RNA positive and negative patients clinically diagnosed with coronavirus disease 2019. Zhonghua Jie He He Hu Xi Za Zhi. 2020; 43 (5), 427-30.
[21] Li C, Su Q, Liu J, et al. Comparison of clinical and serological features of RT-PCR positive and negative COVID-19 patients. J Int Med Res. 2021; 49, 300060520972658.
[22] Korkmaz I, Dikmen N, Keleş FO, Bal T. Chest CT in COVID-19 pneumonia: correlations of imaging findings in clinically suspected but repeatedly RT-PCR test-negative patients. Egypt J Radiol Nucl Med. 2021; 52, 96.
[23] Chen ZH, Li YJ, Wang XJ, Ye YF. Chest CT of COVID-19 in patients with a negative first RT-PCR test: Comparison with patients with a positive first RT-PCR test. Medicine (Baltimore). 2020; 99, e20837.
[24] Nalbant A, Kaya T, Varim C, Yaylaci S, et al. Can the neutrophil/lymphocyte ratio (NLR) have a role in the diagnosis of coronavirus 2019 disease (COVID-19)? Rev Assoc Med Bras. 2020; 66: 746-51.
[25] Zeng ZY, Feng SD, Chen GP, Wu JN. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19: a prospective cohort study. BMC Infect Dis. 2021; 21, 80.
[26] Toori KU, Qureshi MA, Chaudhry A, Safdar MF. Neutrophil to lymphocyte ratio (NLR) in COVID-19: A cheap prognostic marker in a resource constraint setting. Pak J Med Sci. 2021; 37, 1435-9.
[27] Alkhatip AAAMM, Kamel MG, Hamza MK, et al. The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2021; 21: 505-14.
[28] Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020; 84, 106504.
[29] Bedel C, Korkut M, Armağan HH. NLR, d-NLR and PLR can be affected by many factors. Int Immunopharmacol. 2021; 90, 107154.
[30] Tural Onur S, Altın S, Sokucu SN, et al. Could ferritin level be an indicator of COVID-19 disease mortality? J Med Virol. 2021; 93, 1672-7.
[31] Ahmed S, Ansar Ahmed Z, Siddiqui I, et al. Evaluation of serum ferritin for prediction of severity and mortality in COVID-19- A cross sectional study. Ann Med Surg (Lond). 2021; 63, 102163.