Le Infezioni in Medicina, n. 3, 372-391, 2022
doi: 10.53854/liim-3003-6
REVIEWS
Human monkeypox disease (MPX)
Ramadan Abdelmoez Farahat1, Ranjit Sah2, Amro A. El-Sakka3, Amira Yasmine Benmelouka4, Mrinmoy Kundu5, Fatma Labieb6, Rahma Sameh Shaheen7, Abdelaziz Abdelaal8,9,10, Basel Abdelazeem11,12, D. Katterine Bonilla-Aldana13,14, Carlos Franco- Paredes15, Andres F. Henao-Martinez16, Mohammed A. Garout17, Darwin A. León-Figueroa13,18,19, Monica Pachar20, José Antonio Suárez21, Juan David Ramirez22,23, Alberto Paniz-Mondolfi22, Ali A. Rabaan24,25,26, Jaffar A. Al-Tawfiq27,28,29, Hiroshi Nishiura30, Yeimer Ortiz-Martínez13,31, Juan Esteban Garcia-Robledo32, Sergio Cimerman33, Alexandre Naime Barbosa34, Pasquale Pagliano35, Gabriela Zambrano-Sanchez36, Jaime A. Cardona-Ospina37,38, Beatrice Bížová39, Alfonso J. Rodriguez-Morales13,14,37,38,40
1Faculty of Medicine, Kafrelsheikh University, Kafrelsheikh 33511, Egypt;
2Department of Microbiology, Institute of Medicine, Tribhuvan University Teaching Hospital, Kathmandu, Nepal;
3Faculty of Medicine, Suez Canal University, Ismailia 41511, Egypt;
4Faculty of Medicine, University of Algiers, Algiers 16000, Algeria;
5Institute of Medical Sciences and SUM Hospital, Siksha ‘O’ Anusandhan, Bhubaneswar 751003, India;
6Faculty of Medicine, Beni-Suef Univesity, Beni-Suef 62511, Egypt;
7Faculty of Medicine, Benha University, Benha 13518, Egypt;
8Harvard Medical School, Boston, MA 02115, USA;
9Boston University, MA 02215, USA;
10Tanta University Hospitals, 31516 Egypt;
11Department of Internal Medicine, McLaren Health Care, Flint, Michigan 48532, USA;
12Department of Internal Medicine, Michigan State University, East Lansing, Michigan 48823, USA;
13Grupo de Investigación Biomedicina, Faculty of Medicine, Institución Universitaria Visión de las Américas, Pereira, Risaralda, Colombia;
14Latin American Network on MOnkeypox VIrus research (LAMOVI), Pereira, Risaralda, Colombia;
15Hospital Infantil de Mexico, Federico Gomez, Mexico City, Mexico;
16Division of Infectious Diseases, University of Colorado, Aurora, CO, USA;
17Community Medicine and Pilgrims Health Department, Faculty of Medicine, Umm Al-Qura University,
Makkah, Saudi Arabia;
18Facultad de Medicina Humana, Universidad de San Martín de Porres, Chiclayo, Peru;
19Emerge, Unidad de Investigación en Enfermedades Emergentes y Cambio Climático,
Facultad de Salud Pública y Administración, Universidad Peruana Cayetano Heredia, Lima, Peru;
20Medicine Department-Infectious Diseases Service, Hospital Santo Tomas, Panama City, Panama;
21Investigador SNI Senacyt Panamá, Clinical Research Deparment, Instituto Conmemorativo Gorgas
de Estudios de la Salud, Panama City, Panama;
22Department of Pathology, Molecular, and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai,
New York, NY, USA;
23Centro de Investigaciones en Microbiología y Biotecnología-UR (CIMBIUR), Facultad de Ciencias Naturales, Universidad del Rosario, Bogotá, Colombia;
24Molecular Diagnostic Laboratory, Johns Hopkins Aramco Healthcare, Dhahran, 31311, Saudi Arabia;
25College of Medicine, Alfaisal University, Riyadh, 11533, Saudi Arabia;
26Department of Public Health and Nutrition, The University of Haripur, Haripur, 22610, Pakistan;
27Specialty Internal Medicine and Quality Department, Johns Hopkins Aramco Healthcare, Dhahran, Saudi Arabia;
28Infectious Diseases Division, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA;
29Infectious Diseases Division, Department of Medicine, Johns Hopkins University School of Medicine,
Baltimore, MD, USA;
30Kyoto University School of Public Health, Yoshidakonoecho, Sakyoku, Kyoto City 6068501, Japan;
31Department of Internal Medicine, Universidad Industrial de Santander, Bucaramanga, Santander, Colombia;
32Division of Hematology and Medical Oncology, Mayo Clinic, Phoenix, AZ, USA;
33Instituto de Infectologia Emílio Ribas, São Paulo, SP, Brazil; Brazilian Society for Infectious Diseases,
São Paulo, SP, Brazil;
34Infectious Diseases Department, Botucatu Medical School, UNESP; Brazilian Society for Infectious Diseases,
São Paulo, SP, Brazil;
35Department of Infectious Diseases, University of Salerno, Salerno, Italy;
36School of Medicine of the International University of Ecuador, Quito, Ecuador;
37Institución Universitaria Visión de las Américas, Pereira, Risaralda, Colombia;
38Grupo de Investigación Biomedicina, Faculty of Medicine, Fundación Universitaria Autónoma de las Américas, 660003, Pereira, Risaralda, Colombia;
39Department of Dermatovenerology, Second Faculty of Medicine, Charles University, University Hospital Bulovka, Prague, Czech Republic;
40Master of Clinical Epidemiology and Biostatistics, Universidad Científica del Sur, Lima, 4861, Peru
Article received 16 June 2022, accepted 24 July 2022
Corresponding authors
Ramadan Abdelmoez Farahat
E-mail: ramadan.med_2587@med.kfs.edu.eg
Alfonso J. Rodriguez-Morales
E-mail: arodriguezmo@cientifica.edu.pe
SummaRY
Monkeypox is a rare viral infection, endemic in many central and western African countries. The last international outbreak of monkeypox reported outside Africa occurred back in 2003. However, monkeypox has reemerged at a global scale with numerous confirmed cases across the globe in 2022. The rapid spread of cases through different countries has raised serious concerns among public health officials worldwide prompting accelerated investigations aimed to identify the origins and cause of the rapid expansion of cases. The current situation is reminiscent of the very early stages of the still ongoing COVID-19 pandemic. Overlapping features between these, two seemingly alike viral entities include the possibility for airborne transmission and the currently unexplained and rapid spread across borders. Early recognition of cases and timely intervention of potential transmission chains are necessary to contain further outbreaks. Measures should include rapid and accurate diagnosis of cases meeting case definitions, active surveillance efforts, and appropriate containment of confirmed cases. Governments and health policymakers must apply lessons learned from previous outbreaks and start taking active steps toward limiting the recent global spread of monkeypox. Herein, we discuss the status of the current monkeypox outbreaks worldwide, the epidemiological and public health situation at a global scale and what can be done to keep at bay its further expansion and future global implications.
Keywords: Monkeypox, outbreak, Orthopoxvirus, global, infection; human.
INTRODUCTION
Human monkeypox (MPX) is a zoonotic viral disease caused by the monkeypox virus (MPXV). MPXV is a double-stranded DNA virus of the genus Orthopoxvirus of the family Poxviridae known for over half a century but geographically restricted to a limited number of endemic countries throughout Central and West Africa. However, during the last two decades, sporadic reports of imported cases have emanated from North America, Europe, and the Middle East. More recently, in 2022, a multicountry outbreak has determined great concern as the disease is rapidly spreading, especially among young men who have sex with men (MSM), causing the classic vesicular-pustular rash along with other clinical manifestations [1, 2]. Multiple studies have been published in the last few weeks (May through June 2022), in an attempt to decipher the diverse aspects driving the current expansion of the disease. Because it is critical to summarize all available medical information about this reemerging viral zoonosis and make it available for healthcare workers, we have developed the current rapid review article including a comprehensive literature search and analysis to aid in the dissemination of knowledge about this disease [1-107].
Historical background
Monkeypox virus was first isolated and identified in captive cynomolgus monkeys (Macaca fascicularis) in 1958 at a lab in Copenhagen, Denmark, while working on poliovirus vaccine research and development [64, 65]. However, it wasn’t until 12 years later (1970) that the first human case was reported in a pediatric patient from the Democratic Republic of the Congo (DRC) [1, 2]. The zoonotic and epidemiological aspects of MPXV are not well characterized yet partly due to a lack of research, especially before 2003 [3, 64-68]. MPX is common in Central and Western Africa across multiple countries where the virus is endemic. This zoonosis had not been reported outside Africa previous to 2003 [4]. Yet, there are still some doubts as to its true origin, given that the originally infected monkeys described in Denmark back in 1958 were shipped from Singapore and not from Africa [64, 65, 69]. This original report also refers to an earlier outbreak in 1922 in Alto Uruguay, Brazil, that occurred amongst Mycetes seniculus and Cebus capucinus monkeys, who developed typical pustules and died in large numbers during a concurrent pox outbreak, considered at the moment to be smallpox [64, 65, 69, 70]. These studies raise many questions about the natural origin of MPXV in both animals and humans.
So far, two unique clades have been identified in Africa: the West African clade and the Congo Basin or Central African clade [5, 71]. Outside Africa, zoonotic transmission has become one of the main sources of human infection. In 2003, prairie dogs previously infected by rodents, imported from Ghana caused a major outbreak of the disease affecting 71 human subjects. The infection was therefore transmitted to humans, exclusively in a zoonotic route (animal-to-humans), without confirmed human-to-human transmission [72].
Sporadic cases and clusters have also been reported outside Africa between 2003 and 2021 [64, 73]. In 2003, an outbreak of 53 human monkeypox cases was reported in the United States of America (USA) [6]. Singapore reported one suspected case in a returning traveler from Nigeria in May 2019 [7]. Three relatives from the same family who had traveled from Nigeria to the United Kingdom (UK) were also confirmed infected in May 2021 [8]. An additional case of a man who moved from Nigeria to Texas, USA and developed human monkeypox was reported in July 2021 [9]. At that same time infection was identified in another patient who had recently moved from Nigeria to Maryland, USA, that same year [10]. A recent systematic review showed a rise in confirmed cases, particularly in highly endemic regions including Benin, Cameroon, Central African Republic (CAR), DRC, Liberia, Nigeria, Gabon, Ivory Coast, and South Sudan. This rise may correlate with the halting of smallpox vaccination ending the 1970s in multiple countries, which is thought to confer cross-protection against monkeypox [4, 71].
In a recent meta-analysis, the pooled case fatality rate (CFR) worldwide reached 8.7% (95%CI 7.0%-10.8%), which was remarkably higher for the Central African clade as compared to the western clade (10.6% [95%CI 8.4%-13.3%] vs 3.6% [95% 1.7%-6.8%]), respectively. The highest CFR was reported among children (<10 years of age) from 1970 to 1990; however, within the past two decades, the CFR decreased to 37.5% [4]. According to a clinical and epidemiological report during Nigeria’s human monkeypox outbreak in 2017-2018, seven deaths occurred among 122 probable or confirmed cases with a mean age of 27 years [12]. Recently, some studies have suggested that the actual burden of MPX in endemic African countries has been poorly characterized. Also, the diversity and extent of animal reservoirs remains unknown. However, the synanthropic rodent population has probably increased in recent years in Africa, leading to more human-rodent interactions and thus increased transmission of MPXV [74, 75].
Background about the virus structure
Poxviruses synthesize their DNA and RNA in the cytoplasm of the infected cell. Poxviridae is a virus family containing many essential viruses that divided into two groups. There are 16 genera and 16 families (Figure 1). Their host range separates the two sub-families: Entomopoxvirinae, infecting insects, and Chordopoxvirinae, which infects vertebrates. Many viruses in the second group, such as monkeypox, cowpox, and tanapox, cause human sickness. MPXV was discovered in 1958 (described as a pox-like disease in monkeys) and given its name in 1971 [64, 65]. Years later, it was placed in the Orthopoxvirus genus and Poxviridae family. MPXV is a brick-shaped virus with an encapsulated double-stranded DNA genome of about 190 kb and a dumbbell-shaped pleomorphic core of 140-260 nm. Both ends of the genome have tight hairpins. They can create the necessary proteins for transcription and subsequent replication as opposed to many DNA viruses [11]. Viral entry is dependent on cell types and viral clades, and happen following a primary attachment to the cellular surfaces via interactions among different viral ligands and the cellular receptors, for example chondroitin sulfate or the heparan sulfate. Posterior passage through cell membrane is facilitated by a viral fusion effect with cell membrane, or by endosomal uptake through a macropinocytosis-like mechanism involving actin [108, 109].
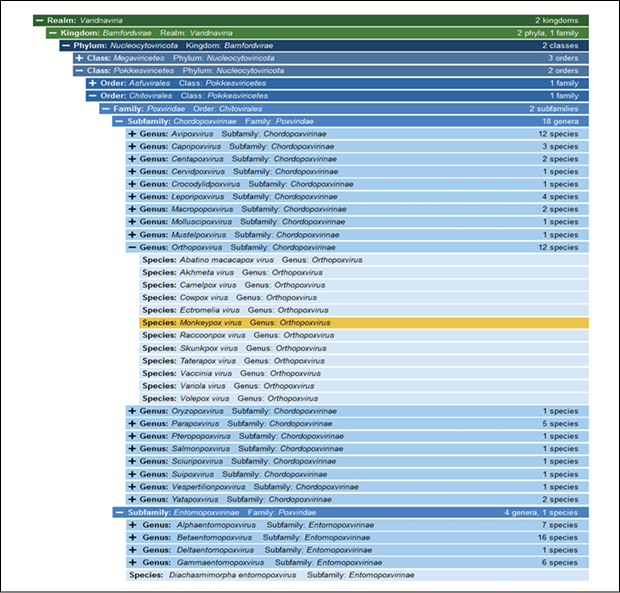
Figure 1 - Poxviruses taxonomy, showing the ubication of monkeypox virus according to the International Committee on Taxonomy of Viruses (ICTV).
Genomic surveillance
Traditionally, MPXV genomic studies have implemented the use of two clades known as the ‘West African’ and the ‘Central African or Congo basin’ clades. However, in order to implement a non-discriminatory and non-stigmatizing nomenclature system, some authors suggest the use of alphanumerical clades, nomenclature already implemented by Nextstrain (Figure 2). Genome sequences from the current 2022 outbreak have now been made publicly available from different countries such as Portugal, Spain, France, Switzerland, Italy, Slovenia, Netherlands, Germany, United Kingdom, Israel, United States of America, Canada and Brazil. The molecular epidemiology landscape of the current multi-country outbreak suggests most likely a dual origin, most of the sequences cluster on the B.1 and A.2 clades, the B.1 clade seems to be an emergent clade that diverged from A.1. (2018-2019 outbreak) with representatives from most of the countries with MPXV cases (Figure 2). The A.2 clade with only three represented genomes, is a clade that diverged from the hMPXV-1A ancestral clade circulating in 2018-2021 (Figure 2). Interestingly, all of the B.1 genomes show the APOBEC3 induced mutations. These mutations are unique or shared in the emergent lineage, warning for future studies to examine if this is the source of variations from the recent outbreak. Large-scale genomic surveillance needs to be strengthened in order to help discern the origin and potential transmission routes leading to the spread of the of the virus in the current outbreak [100-105].
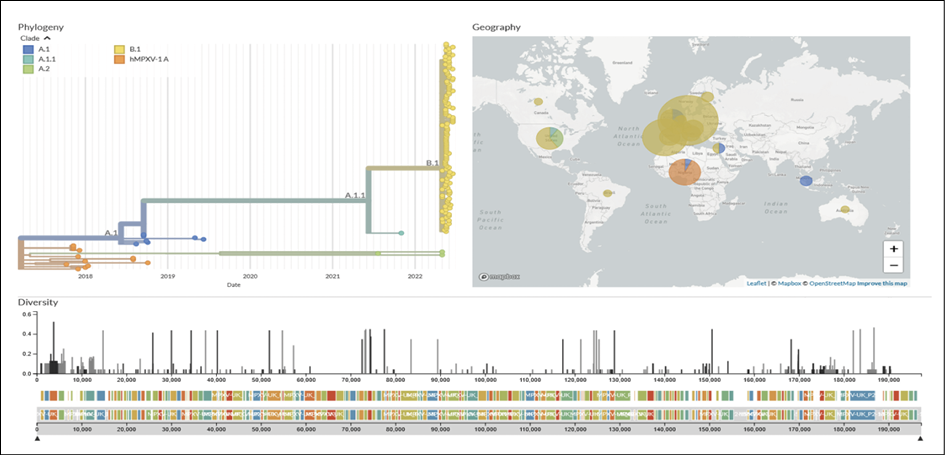
Figure 2 - Genomic epidemiology of monkeypox virus (145 genomes sampled between 2017 and 2022). Built with nextstrain/monkeypox, maintained by Nextstrain team, and enabled by data from GenBank. Source: https://nextstrain.org/monkeypox/hmpxv1
Animal hosts in natural infection
MPXV is infective in a wide range of lab animals, and various species and exposure modalities have been implemented to create several animal models. MPXV is one of the poxviruses heavily employed to generate little animal models through various exposure routes due to the variola virus’s inability to develop animal models and the subsequent illness symptoms shared with humans. Different exposure routes make inbred wild-derived mice, STAT1-deficient C57BL/6 mice, prairie dogs, African dormice, and ground squirrels vulnerable to the MXPV [12].
The range of genera, species, families, and orders of mammals affected by MPXV is wide (Table 1), including non-human primates, arboreal and terrestrial rodents (Figure 3), and other animals. Among them, spillover between different families seems to be shared, especially in specific ecological settings. Therefore, the list of affected species probably will be higher and needs in-depth assessment under the current outbreak conditions to understand if other non-African rodents are susceptible to MPXV. Some of the susceptible listed species (Table 1) are already present outside Africa, and the risk of infection and enzootic cycle establishment is critical at the moment [64-66].
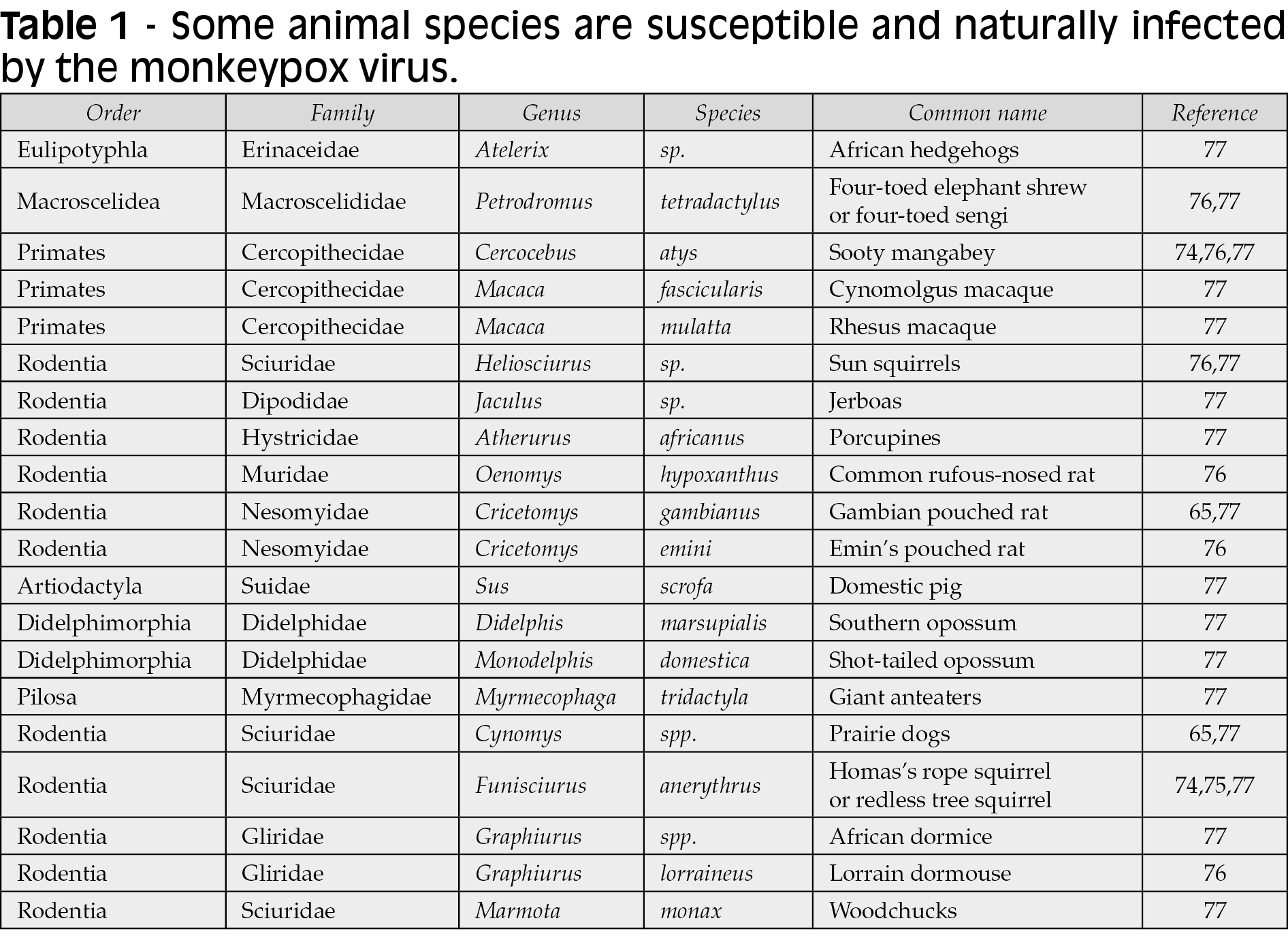
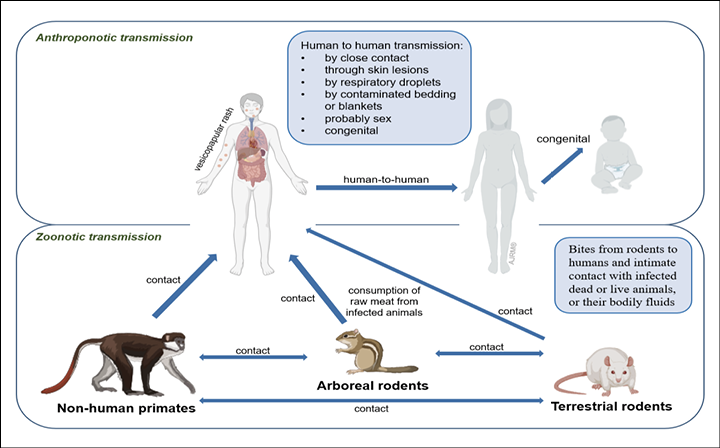
Figure 3 - Transmission routes of monkeypox.
Infection and transmission routes
Skin is considered the primary source of infection (Figure 3) [13]. Although respiratory droplets are thought to transmit disease from person to person, the US Centers for Disease Control and Prevention (CDC) states that this approach needs prolonged face-to-face contact due to the droplets’ inability to travel a long distance (Figure 3). While monkeypox is not sexually transmitted through sperm or vaginal secretions, authorities say the most recent outbreak is due to male-to-male sexual intercourse [14]. Recently, MPXV has been detected in seminal fluid, genital and rectal lesions, and feces and saliva from confirmed cases in Italy [78]. Monkeypox spreads through bites from rodents to humans and intimate contact with infected dead, live animals, or bodily fluids (Figure 3). Human-to-human transmission occurs by close contact with infected lesions, respiratory droplets, or bodily fluids (Figure 3). The precise MPXV host reservoir species is not known, however it is thought to be small rodents like prairie dogs, squirrels, rabbits, and others, with primates (monkeys and humans) considered as accidental host (Table 1) [15]. Congenital infection may occur in Africa, but there is a lack of confirmatory studies (Figure 3) [66, 79].
Clinical findings
Monkeypox is a self-limiting disease, and the duration of symptoms is approximately 2 to 4 weeks [31]. The incubation period of monkeypox is usually 6-13 days but can range from 5 to 21 days in some cases (Figure 4). Short after the incubation period, monkeypox infection undergoes two phases or periods (Figure 4); the invasion phase and skin eruption (rash phase) (Figure 5).
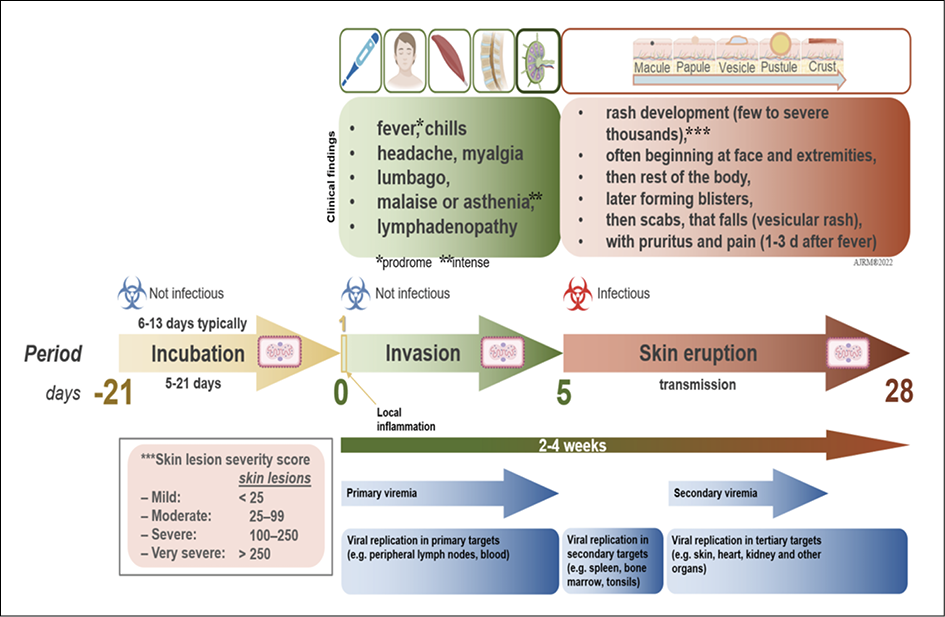
Figure 4 - Clinical evolution of MPX.

Figure 5 - Cutaneous lesions in a patient with confirmed MPXV infection from Prague, Czech Republic [80]. Patient has HIV and syphilis coinfection.
Initial clinical findings of human monkeypox are very alike those of smallpox, chickenpox, and measles. It begins with a prodromal phase, that can include fever, headache, myalgia, and severe asthenia (Table 2). Early in the disease, lymphadenopathy caused by monkeypox is what differentiates it from smallpox. Splenomegaly and hepatomegaly can also be found in these patients, as MPXV replicates in different lymphatic tissues and other organs (Figure 4) [81, 82]. The rash begins on face and extremities, including palms and soles in 75% of cases within 1 to 3 days of fever appearance (Figure 4) [83]. Subsequently, during the cutaneous rash phase, oral mucous membranes, genitalia, conjunctivae, cornea, and the lungs may also be involved [13].
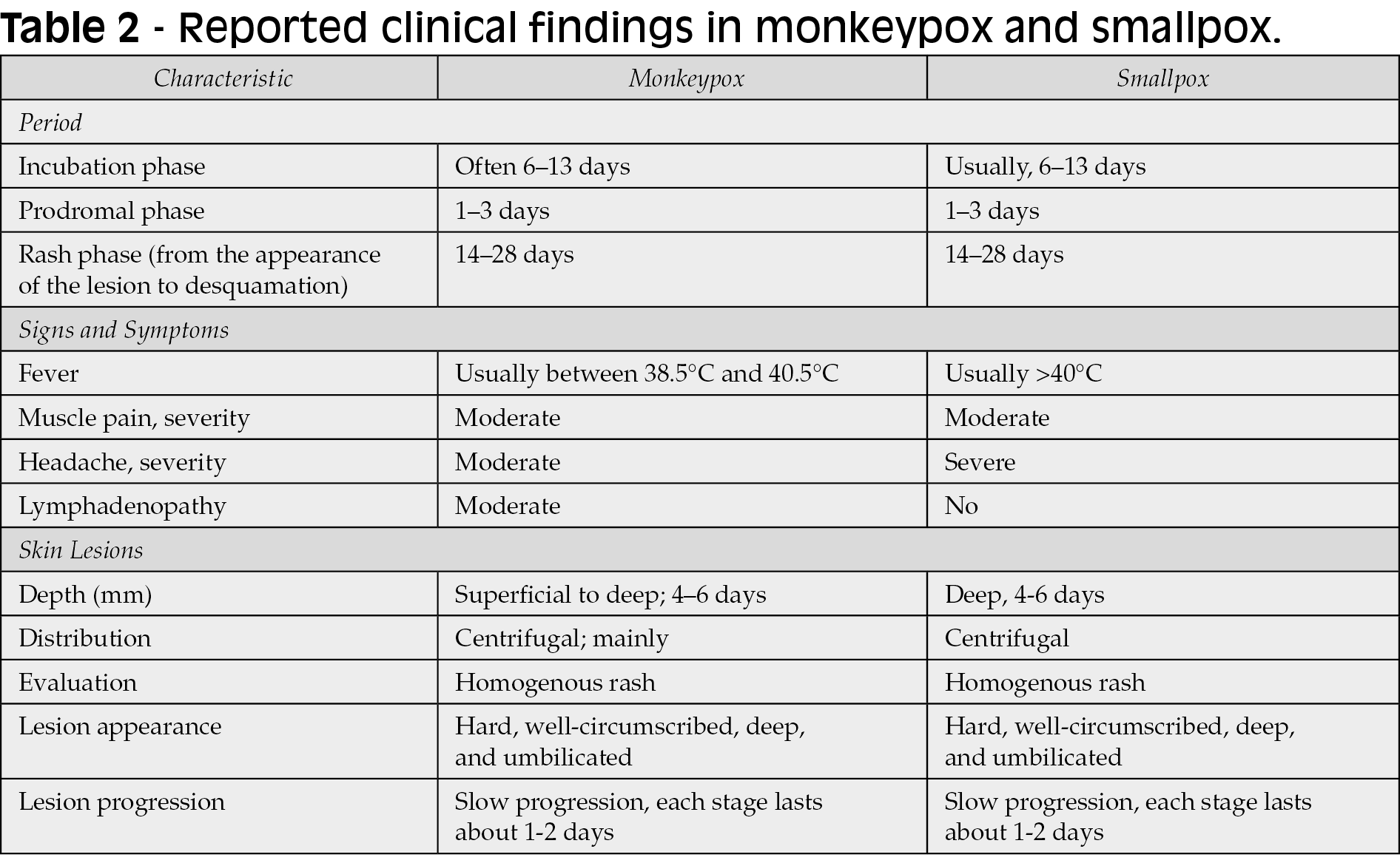
Additionally, cutaneous lesions may evolve into raised bumps and papules, which subsequently blister, resembling chickenpox [16, 17]. Lesions can be filled with a white fluid and develop into pustules an abscesses, which later breakoff and scab [18]. Pustular lesions remain for 5 to 7 days before crust formation, in which a second febrile period along deteriorating conditions may follow. Finally, crusts develop and desquamate after 1-to-2 weeks (Table 2) [13]. One of the most important clinical characteristics that differentiates monkeypox from other entities in the differential diagnosis is that all the skin lesions evolve monomorphically during each phase, as opposed to varicella for example, which can present with asynchronous lesions such as papules, vesicles and crusts at the same time [91]. It its worthy to note is apparently seem that 2022 cases may present beginning just with genital ulcers [90, 106].
The vesicular-pustular rash is the clinical hallmark feature of monkeypox, largely impacting the infected individual (Table 2) (Figure 4). Lesions are characterized by progressive ulceration, necrosis, and epithelial hyperplasia (Figure 4). Dermal healing generally proceeds through inflammation, proliferation, and remodeling phases. Risks for secondary infection have not been the subject of focus, but they can furt her contribute to the development of cellulitis or sepsis [19, 20]. Any rash developing in the genital or perianal area and presumed to be monkeypox should be thoroughly assessed since it may overlap and mimic a variety of other sexually transmitted diseases [21]. More recently, corneal scarring has been reported to be one of the most common complications of monkeypox infection in the US. In the province of Tshuapa (DRC), about 25% of confirmed MPXV cases reported “conjunctivitis” as a disease symptom [22-25]. Another recent consideration is the possibility of coinfections (e.g., Human Immunodeficiency Virus [HIV], syphilis, and other sexually transmitted infections) and how this may influence the clinical course of disease [80].
Anyone with a fever and subsequent pustular rash after visiting an endemic area of monkeypox, such as the DRC or Nigeria, should be screened for monkeypox (Figure 6). The laboratory-confirmed infected patient should immediately be isolated. Additionally, the CDC should be notified to begin investigations and trace close contacts exposed to the index case, either after arrival or during travel in the USA [15]. Same scenario applies to other local center for disease control. The World Health Organization (WHO) has recommended that patients suspected of infection with MPXV should be investigated, confirmed, and isolated until lesions resolve, meaning have crusted and the scab has fallen off. Re-epithelization usually forms underneath scabbed tissue. It is recommendable covering the lesions with a bandage, sheet, or gown so that others can avoid potential contact with the lesions (Figure 5).
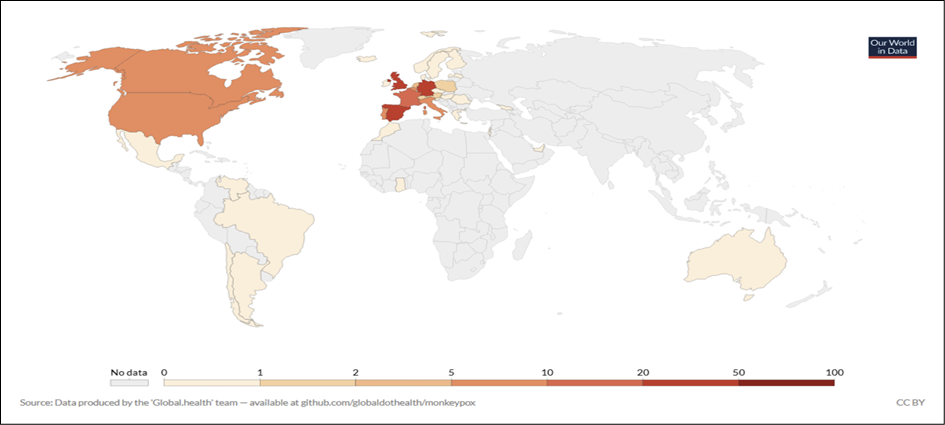
Figure 6 - Geographical distribution of monkeypox cases in 2022, up to June 18, 2022. From: https://ourworldindata.org/monkeypox.
Severe forms can be observed in the pediatric population, people living with HIV/AIDS (PLWHA), and other immunosuppressed patients [78, 80, 84-86]. Complications are secondary infections, pneumonia, sepsis, encephalitis, and keratitis associated with vision loss, among others [32, 88].
Monkeypox and HIV
Even though it is reasonable to assume that due to underlying immunosuppression, the course of monkeypox should be more severe in PLWHA, the effects of monkeypox in this patient population are yet to be determined. Reports emanating from Africa during several local outbreaks, particularly in Nigeria, where cases of coinfection have been described show variable results [92-94]. In one study, including 118 cases of MPXV in which there were seven casualties, four of these were HIV patients (three cases cited as advanced HIV without ART). Another study including 40 MPXV patients noted that at least nine patients had HIV (of which seven had at least a high viraemia and low CD4 counts) [2, 95]. Outside Africa, evidence is unreliable, as HIV status was not recorded in most past outbreaks [87]. However, after reviewing these studies, one could assume that an uncontrolled or advanced HIV infection could pose a risk factor for prolonged MPXV shedding, severe disease, and/or mortality [96]. As of this date, there are no specific recommendations regarding managing HIV patients with risk of exposure to MPXV beyond vigilance regarding clinical presentation and history of exposure. However, one could advise caution in patients with low CD4 counts (<200 cells/mm3), (e.g., AIDS diagnosis in the prior six months), and persistent HIV viraemia (e.g., >200 copies/mL) [97]. Regarding immunization, non-replicating smallpox vaccines could be used in this population. For example, the Imvanex (Bavarian Nordic) MVA-BN vaccine has been studied in PLWHA with CD4 counts greater than 100 cells/mm3. However, vaccine efficacy is yet to be ascertained in patients with uncontrolled viremia o low (<100) CD4 counts. As such, it is recommended to seek specialized advice to evaluate the need for immunization in this population [98]. As more detailed information becomes available, more solid recommendations would be developed regarding this vulnerable population.
Differential diagnoses of monkeypox
The differential diagnosis of monkeypox includes a wide range of non-infectious and infectious conditions, in particular those DNA and RNA viruses that may exhibit cutaneous manifestations (Table 3). This large list includes smallpox, cowpox, tanapox, molluscum contagiosum (Pox viruses); herpesviruses, such as HSV-1, HSV-2, chickenpox (varicella), zoster (shingles) (Figure 7), exanthem due to CMV or EBV, HHV-6, -7 and -8; adenovirus, human papillomavirus (HPV) and parvovirus B19 (Table 3) among others. Also, RNA viruses may course with rash and other cutaneous manifestations, including paramyxoviruses such as measles (Figure 7), mumps, AIDS dermatitis, hand-foot-mouth disease (HFMD) (also compromising buttocks) (Figure 7), and exanthems due to enterovirus and Coxsackie viruses (Figure 7), echoviruses, and rubella. Bacterial diseases, such as syphilis, should always be considered (Figure 7). Across tropical countries, multiple arboviruses may display rashes, with or without pruritis, with dengue, chikungunya, Zika, yellow fever, West Nile virus, Japanese encephalitis, tick-borne encephalitis being the most common of these. Other rodent-borne viruses, such as mammarenaviruses, may also exhibit skin manifestations, e.g., South American hemorrhagic fevers: Argentinian (Junin), Bolivian (Machupo and Chapare viruses), Brazilian (Sabia), and Venezuelan (Guanarito) [83]. Ectoparasitic diseases, such as scabies and cutaneous larva migrans coursing with serpiginous or serpentine papules and / or multiple erythematous papules can be a challenging disease mimicker (Figure 7). Some of these conditions may also present as coinfections [80, 99]. Drug hypersensitivity / Stevens-Johnson syndrome should be entertained in the right clinical scenario (Figure 7).
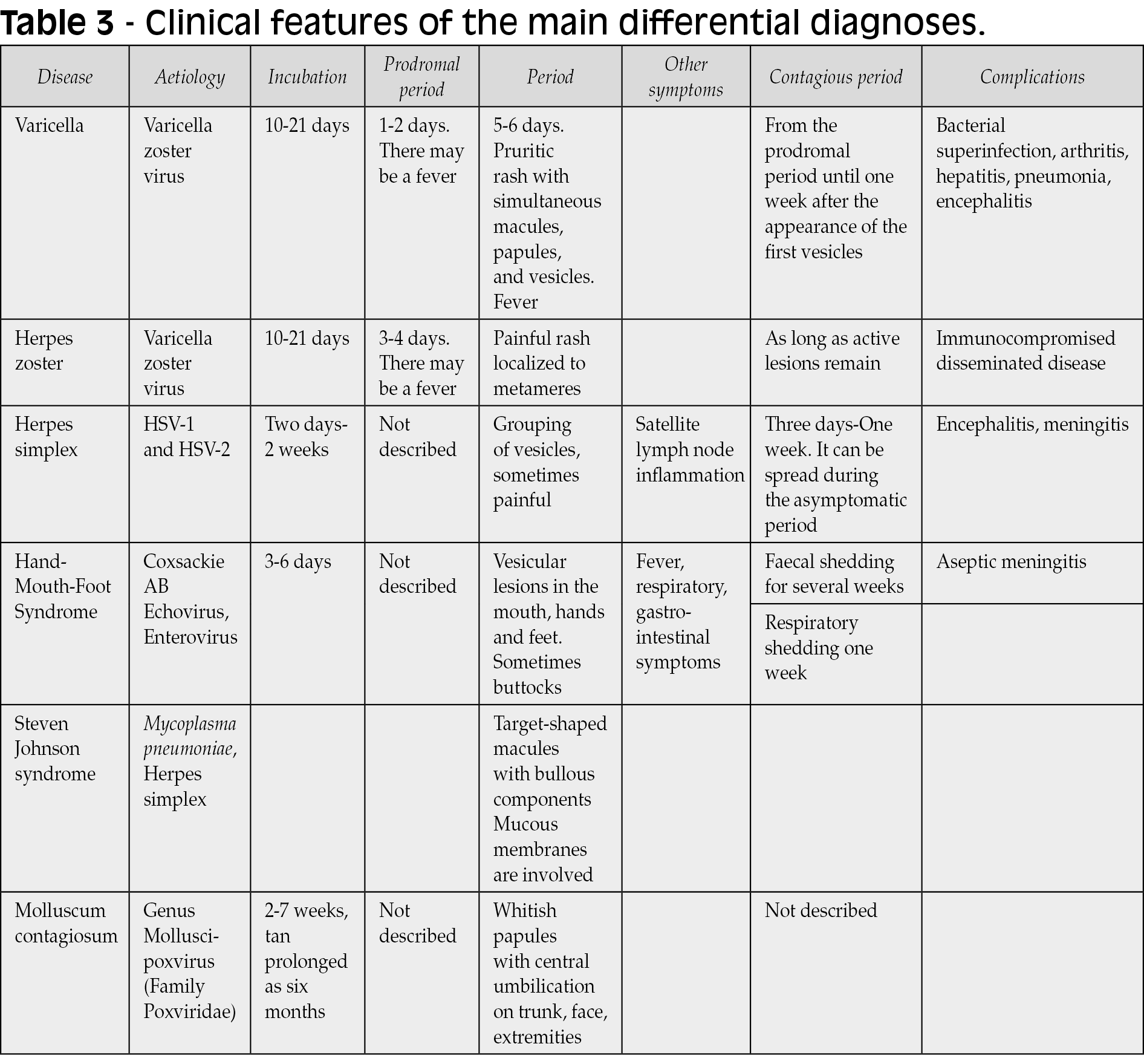
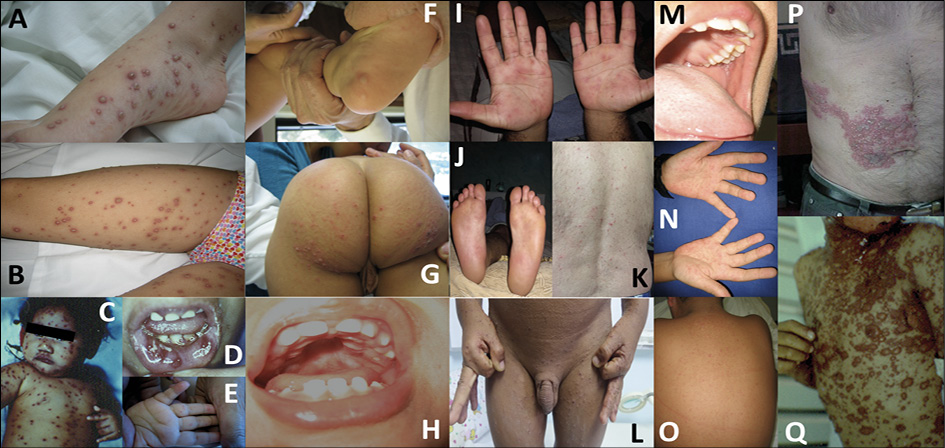
Figure 7 - Clinical differential diagnosis of monkeypox; Varicella (panels A and B), Herpes simplex, disseminated (panel C) and herpetic gingivostomatitis (panel D), foot-and-mouth disease due to Coxsackievirus (panels E, F, G and H), secondary syphilis (panels I, J and K), scabies (panel L), measles (panels M, N, and O), metameric Herpes zoster (panel P), and Stevens-Johnson syndrome (panel Q) (Photos took by Dr. J.A. Suárez, with consent).
Available treatments and vaccines for monkeypox
No licensed treatment or proper evidence-based guideline is currently available for treating human monkeypox. Thus, clinical management aims to provide symptomatic treatment, manage complications, and prevent long-term sequelae (Table 4). Recently, the WHO has published an interim guideline for clinical management [88].
The US FDA has approved tecovirimat and brincidofovir for smallpox treatment [27-29]. None of these drugs has been tested on humans in phase 3 efficacy trials, but both have shown efficacy against other orthopoxviruses in animal models, including monkeypox [29]. JYNNEOS®, also known as Imvamune® or Imvanex®, has been approved in the USA to prevent monkeypox and smallpox and is currently used in context of occupational exposure [30, 89]. Previous data from Africa shows that this smallpox vaccine is 85% effective in preventing monkeypox; nevertheless, this needs further assessment [30]. Another vaccine, the vaccinia Ankara has also been modified for clinical use. Unlike live vaccine preparations, it does not have a risk of spreading either locally or disseminated [19]. Clinical efficacy trials have also highlighted the safety of this vaccine by stimulating antibody production in patients with atopy and compromised immune systems [20].
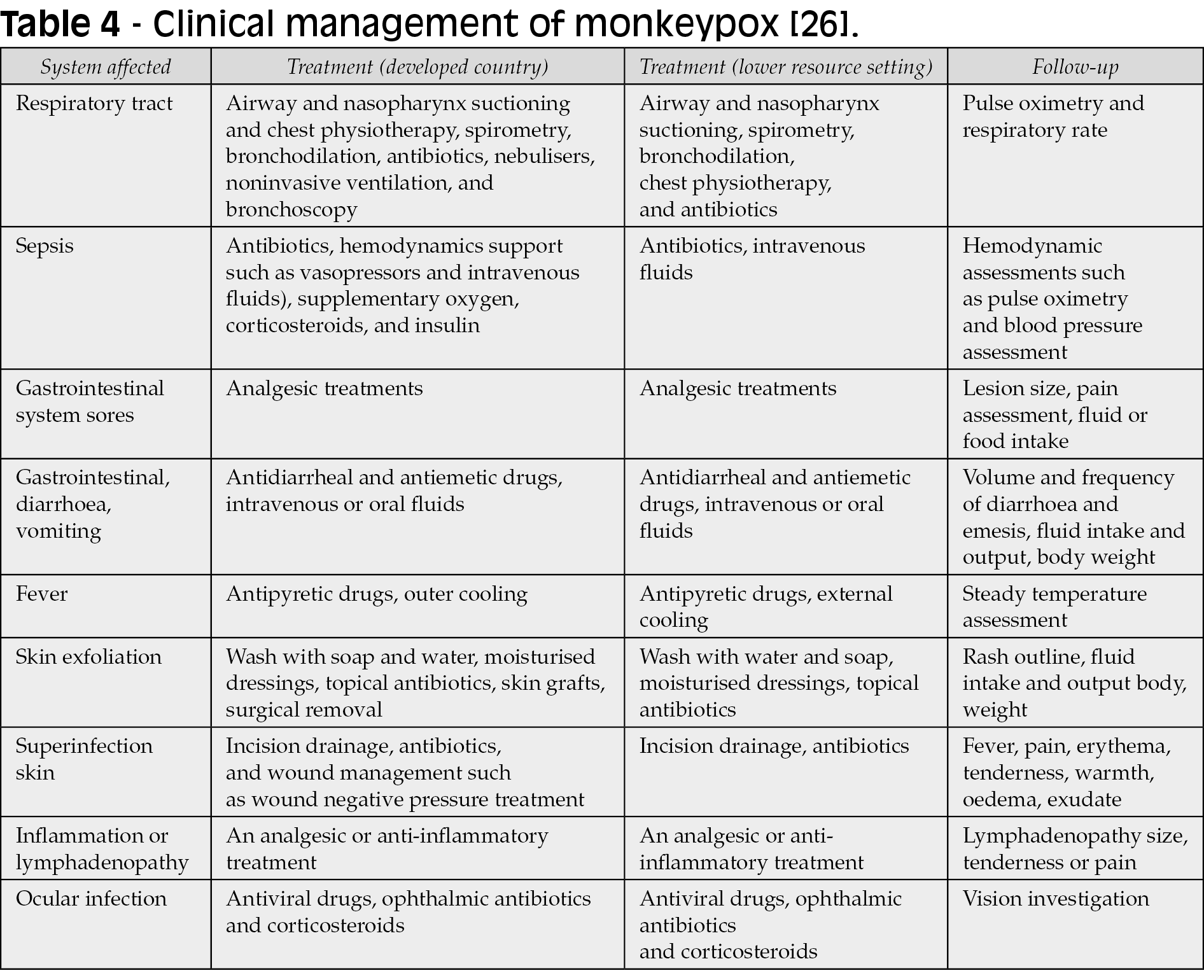
Antiviral drugs such as tecovirimat, cidofovir, and brincidofovir can be considered mainly for those with severe symptoms or who may be at risk of poor outcomes, such as those with immune suppression. In addition, vaccines such as JYNNEOS® and vaccinia Ankara® can be used for monkeypox, but they are not yet widely available. The WHO also recommends that some countries may hold smallpox vaccine products for use according to national guidance [31]. For example, in a recent report from the UK, brincidofovir (200 mg, 1-2 doses) and tecovirimat (600 mg twice daily for two weeks) were used in confirmed cases attended between 2018 and 2021 [29]. Potential drug-drug interactions can occur in patients on antiretrovirals (e.g., cidofovir has high nephrotoxic potential, so its use should be avoided with nephrotoxic antiretrovirals, such as tenofovir-disoproxil), therefore, and should be encouraged to check interactions with HIV drugs in the Liverpool site: https://www.hiv-druginteractions.org/checker.
In cases of ocular involvement, steroid drops utilized to manage inflammation may worsen disease course and further contribute to corneal damage and viral persistence; however, simple local therapies such as enhanced lubrication or topical antibiotics could be considered [21]. In the lungs, bronchopneumonia is a rare complication of MPXV. Many studies reported the accumulation of virus-infected aerosols in the trachea leading to respiratory infection and even death. This has been studied in a large cohort of animals, in which secondary bacterial infection was noted in one animal unlike the rest [22, 23].
Early detection of the disease will help enhance public health control measures. In absence of currently available licensed and effective drugs for monkeypox, immediate vaccination is the most effective intervention for public health protection once diagnosis has been confirmed. During the 2003 outbreak in the United States, the CDC published case definition criteria to accurately diagnose human monkeypox. The confirmed human monkeypox case requires laboratory evidence, unlike the clinical and epidemiologic criteria, which may differ by situation and geographic location [24, 25]. PCR analysis of vesicle fluid or scabs can be performed for laboratory confirmation during disease activity. After disease resolution, testing for varicella virus IgM can be performed [32]. The CDC has also crafted a protocol to differentiate between human monkeypox infection and smallpox to determine whether patients will require additional investigations [19, 33].
Monkeypox is usually a self-limited disease, and most conditions resolve in around 3-4 weeks after the onset of symptoms. Patients do not risk infecting others after all crusts desquamate [34]. The infected individual should wear a surgical mask, be isolated, and cover the lesions until all crusts desquamate and the formation of a new skin layer ensues [19]. For individuals exposed to the virus or who have close contact with infected patients, their temperature and symptoms should be assessed twice daily for three weeks. In some cases, post-exposure vaccination is recommended, especially if the contact was between infected injured skin, scabs, mucous membranes, body fluids, or respiratory droplets. All are at a high-risk exposure, and vaccination is required. Within four days after exposure, vaccination may halt the onset and progression of disease. Within 14 days, vaccination may reduce disease severity, according to data from the CDC [35, 36].
The current situation during the ongoing COVID-19 pandemic
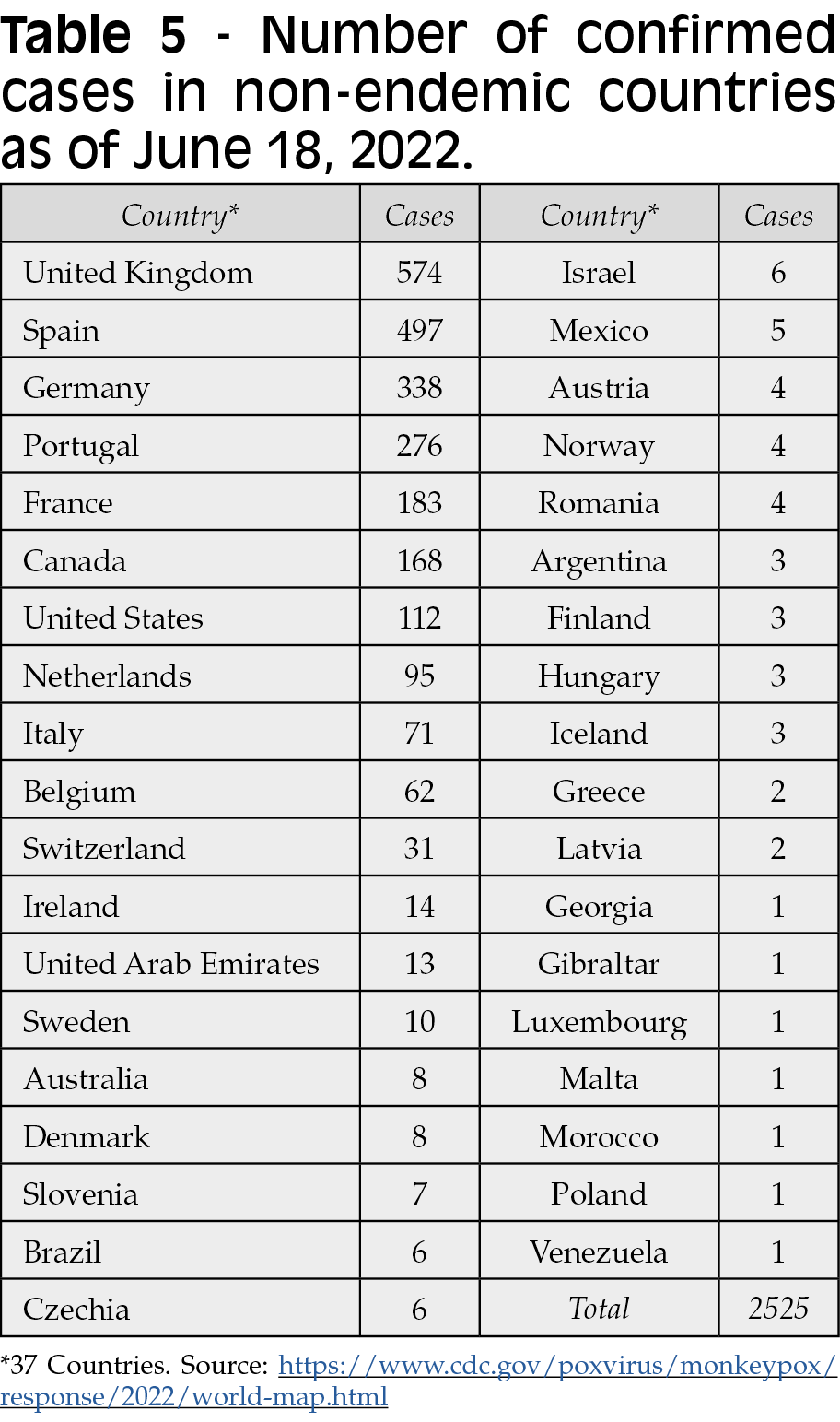
A number of well-documented monkeypox cases have been reported throughout the COVID-19 pandemic. One case, a returning traveler from Canada to Massachusetts was reported as the first case in the US in May 2022. Two cases within the same family whom had been infected and later confirmed in the UK on May 14, 2022 followed. These two cases had no apparent contact with any previously imported case from Nigeria. Since then, many other clusters of human monkeypox have been reported worldwide, many of them with no travel link to endemic countries. As of May 25, 2022, 219 monkeypox cases from non-endemic countries worldwide have been reported, with a total of 118 confirmed cases from twelve European Union/European Economic (EU/EEA) Member States. These numbers increased to 2525 confirmed cases up to June 18, 2022 in 37 countries (Table 5) [37]. The reported cases are mainly but not exclusively represented by young men who have sex with men [31]. Interestingly, no deaths have been reported until now, but fatal case was investigated by June 18, 2022 in Brazil [37]. It is important to remember that in Africa deaths associated with MPX infection have been reported over the time. Up to June 18, 2022, there has been a rapid and broad geographic distribution of monkeypox cases worldwide (Figure 6).
The CFR of monkeypox ranged from 0 to 11% in the past. Recently it has been around 3-6% [38]. The Johns Hopkins University data has reported that the COVID-19 CFR is 1.2% in the USA only, but it is different worldwide [37]. CFR is considered higher in children but needs further definition. However, the COVID-19 preventive measures are helpful against monkeypox transmission [31]. There is a potentiality of the coinfection between MXPV and SARS-CoV-2, especially during the ongoing COVID-19 pandemic [39, 40].
Another interesting aspect for epidemiological purposes is the discussion regarding the basic reproductive number. Previous analyses, using data from the DRC (1980-1984) suggested for the Congo basin clade of monkeypox at that time a R0 of 0.32 (uncertainty bounds 0.22-0.40) [107]. However, using 85% for vaccinia efficacy (meaning effective coverage) against monkeypox and the model, authors suggested that the calculated R0 for monkeypox would be 2.13 (uncertainty bounds 1.46-2.67). Currently, with the 2022 global estimations of elderly population, the one to be covered (in an assumed ~85% for monkeypox due to smallpox vaccination before 1980), of 9.77% (776.9 million people), the R0 for monkeypox in 2022 would be between 1.7-2.2, explaining the current spreading. R0 in influenza is 1.3, COVID-19 2-3, and measles 15-18.
Possible causes and risk factors behind the 2022 monkeypox multicountry outbreak
Monkeypox is the most common cause of human Orthopoxvirus infection, after the eradication of smallpox in the 1980s, with most cases reported from West and Central Africa. Therefore, identifying key risk factors is crucial to prevent amplification of the current outbreak. Studies have shown that living in the same household, sharing the same bed or room, and eating or drinking from the same dish were risk factors for human-to-human transmission of the virus [4, 11]. In contrast, outdoor sleeping, visiting or living nearby a forest are risk factors for zoonotic transmission of monkeypox [41, 42]. Intriguingly, assisting with hygiene and clothes washing of an infected patient has failed to correlate with an increased risk of transmission of monkeypox [18]. According to the WHO, close contact with infected persons is the most significant risk factor for monkeypox infection [38]. Thus, the infected person’s healthcare workers and household members are at greater risk of contracting the disease. WHO has also reported that unprotected contact with sick or dead animals, including their meat, blood, or other parts, can also be a potential risk factor for transmission of this virus [38]. In a study published in 1988, most of the cases corresponded to children under ten years of age [43, 44].
The halting of smallpox vaccination may be a risk factor for monkeypox infection [45]. Data suggests that males are at higher risk of the disease, but this may be explained by the frequent contact of males with wild animals in endemic regions [45]. Smallpox and MPXV are closely related [46]. Vaccination against smallpox shows about 85% cross-protection against the MXPV [30]. The Global Commission for the Certification of Smallpox Eradication (GCCSE) did not support the smallpox vaccine continuation for monkeypox zoonosis prevention after finishing the campaign for the smallpox eradication, based on the epidemiological data at that time [47, 48]. Current data confirms that this incidence has increased after the discontinuation of smallpox vaccination [5, 45]. Monkeypox cases’ resurgence may be promoted by other factors, including waning immunity and rapid deforestation of endemic regions [49, 50].
Furthermore, the virus’s genetic evolution might contribute to monkeypox zoonosis resurgence. The monkeypox’s viral genome analysis showed a gene loss in 17% of 60 different human samples, variation that may be tied to human-to-human transmission [51].
Current situations of health care systems and expected response to monkeypox
Our current knowledge on monkeypox is mainly limited to the sporadic case and outbreak reports. Therefore, the response to the current situation is challenging. While efforts on genomic surveillance are still ongoing, results from a wider collection of genomes would help experts to identify and understand the chains of transmission worldwide [49]. Furthermore, response strategies against the resurgence of MPXV have not been well documented. In general, multidisciplinary efforts should be implemented to enhance public health preparedness and establish active surveillance initiatives, mainly in low-income countries. Infection control policies should be promoted in public hospitals. Healthcare providers’ training should also be encouraged to manage individuals with possible monkeypox infection [52]. It is also necessary to provide vaccinations, diagnostic tests, and antiviral drugs. However, such precautions can be hard to implement in resource depleted settings.
The previous experience in the USA facing monkeypox is limited but demonstrated the importance of infection control principles, including index patient isolation and contact tracing [52]. The Nigerian CDC’s response to the monkeypox outbreak may also serve as an example of implementing human-animal disease surveillance and response systems [53]. In addition, this experience may improve surveillance capacity, disease prevention, clinical practice, data collection, preparedness, and laboratory diagnostics implementation in other African countries, particularly those suffering from a lack of resources [54].
Monkeypox defies public health authorities, particularly in regards to laboratory capacities, surveillance, disease treatment, and the lack of knowledge and experience among health care workers to recognize, diagnose, and treat monkeypox; makes disease control even more challenging [55]. Monkeypox cases are occasionally more severe than usual, with some deaths reported in Central and West Africa. However, authorities have emphasized that the risks to the public are very low, and we are not facing a serious outbreak. Severe cases are common among children and are related to patients’ low immunity, health status, nature of complications, and the extent of virus exposure. Today, scientists seek to understand how a less-lethal virus relative to smallpox has cropped up in many populations worldwide. The severity of the monkeypox virus lies in its broad ability to spread. Suppose a more virulent strain of monkeypox was introduced to a population with no previous exposure and immunity to Orthopoxviruses. In that case, this would provide an opportunity to breach into the population, leading to an expansion of cases at epidemic proportions; which is why scientists are alert [38, 55, 56]. Many authorities are discussing available preventive measures given the disease’s alarming spread. For example, in Canada, regular meetings are organized by territorial, provincial, and federal chief medical officers of health to discuss the current situation of this emerging zoonosis [54]. There are also growing efforts to build a global research agenda for monkeypox [54]. Furthermore, the WHO appeals to experts to study and release recommendations about the vaccination necessity for monkeypox, which may be helpful for vulnerable populations, close contacts, and healthcare providers.
Recommendations and future implications
Message to policymakers
When facing a potential monkeypox outbreak, we must learn from history, like the recent COVID-19 pandemics. Despite the decreasing number of cases and deaths, the COVID-19 pandemic continues to evolve as it transits to what appears to be an endemic fate for the SARS-CoV-2 virus. Halting an outbreak before bursting out of control, notably an outbreak with an unexplained widescale spread like monkeypox, should always be a priority when such a risk is looming on the horizon. Discovering cases of monkeypox in many countries without identifying an apparent cause should be a wakeup call for governments and policymakers to start setting plans and taking serious steps toward handling this outbreak [56]. That begins by raising awareness about the disease and establishing educational campaigns to the general public and healthcare workers regarding disease manifestations, transmission, and prevention. In addition, it is vital to support the healthcare professionals in providing the means of protection, whether by providing personal protective equipment (PPE) or vaccinations, especially in the underserved areas. Governments should also double their research efforts on finding an explanation for the unprecedented and widescale spread of the virus. Whether it is caused by genetic mutations leading to the emergence of a novel lineage or because of an increased trade of exotic animals, it is imperative to identify the causes of the problem if we want to tackle it before things wreak havoc. Affected countries should also consider implementing vaccination campaigns for those most vulnerable groups [31].
Veterinarians also have a critical role in the current outbreak as animals act as reservoirs for the virus [52, 57-59]. Therefore, implementing screening programs for animals, particularly the ones imported from monkeypox hotspots like countries of central and western Africa, can prove effective in preventing animal-to-human transmission.
Message to healthcare professionals
Healthcare professionals have a daunting challenge on their hands facing that virus. They are responsible for diagnosing and treating infection, educating their patients about the symptoms and how the virus can transmit from one patient to another, and keeping themselves protected from infection. Monkeypox, being such a rare disease, was rarely the first probability to come to mind when seeing a patient with a rash. As a result, very few physicians have hardly seen a case of monkeypox in their life. However, with the current situation where many countries have an unexplained surge of cases, all in a matter of days, physicians should take care that cases of rash could be monkeypox. A group that has been particularly vulnerable to monkeypox is MSM, PLWHA, and the LGBTQI+ community [60]. However, medical personnel should be alert to any case of rash and consider the possibility of monkeypox regardless of the gender or sexual orientation of the patient.
Healthcare workers must familiarize themselves with the clinical presentation of this recently reemerging virus and be able to act accordingly. Physicians and nurses must commit to protecting themselves in case of a monkeypox case because any morbidity to the healthcare team will only burden the healthcare system further in a time when every medical professional is needed in battling a potential outbreak in their community. Every medical team should feel responsible and appreciate their role in these circumstances, which might not be much different from the COVID-19 pandemic.
Preventive strategies
Preventive strategies need to be deployed to further combat the spreading of the virus. That can be done by raising public awareness about the topic, offering protective equipment to medical personnel terms as gloves, masks, and protective clothing, isolating infected individuals, preferably in unfavorable pressure rooms, and providing immunizations for high-risk groups like healthcare personnel who encounter cases of monkeypox, laboratory workers, veterinarians, and contacts of monkeypox patients.
Improving the diagnostic capabilities
Improving diagnostic capabilities is also a significant aspect that should be thought of. Previously, in similar circumstances to the current outbreak, educational seminars and workshops have proven helpful for healthcare professionals [61]. Especially since monkeypox is rarely seen in many countries currently affected, medical personnel should be able to recognize the clinical presentation of the disease, its routes of transmission, differential diagnosis, and its management. In addition, they should be trained on sample collection and transportation while maintaining appropriate infection control measures. Polymerase chain reaction (PCR) is a reliable diagnostic tool for the MXPV [4]. Implementing a screening program targeting vulnerable groups like patients’ contacts and MSM individuals can contribute to limiting the spread. Thus, it is imperative to have PCR-ready units available in the event of suspecting a monkeypox case in a community, especially the underserved ones where there is poor medical care and transportation.
Surveillance and reporting
Surveillance programs should be set on high alert with a clear action plan for dealing with reported monkeypox cases. Healthcare workers should actively report any suspected case with sufficient details so contacts can be tracked and the source of infection can be identified. The general public should also be encouraged to report any suspected case of monkeypox to their local healthcare units and seek medical help. Furthermore, molecular surveillance of the monkeypox virus can help develop and monitor public health interventions. The currently circulating virus clade in Europe appears to be from West Africa; however, further studying of the virus genome can help identify the causes of this outbreak, describe the local transmission networks, and investigate the connection, if any, between this outbreak and other outbreaks like COVID-19 [62].
Hospital hygiene practice
Hospitals admitting monkeypox patients should follow strict infection control measures to prevent the spread of the virus and infecting other patients or medical personnel. If possible, a patient with monkeypox should be isolated. Patient movement out of the isolated place should be minimized, wear a face shield and have any skin lesions covered with a gown or a sheet while outside. Healthcare staff should have their full PPE on whenever they are inside the patient’s room or interacting with the patient. Wet cleaning is the preferred method of cleaning the patient’s room. Other methods like dry dusting and vacuuming should be avoided [63].
Take-home messages
The reemergence of the MXPV and its widespread across borders is concerning. Raising awareness of the healthcare professionals and the general public, establishing surveillance programs, and providing early diagnosis and management are critical in facing this outbreak. History has taught some valuable lessons in handling such outbreaks, and we must learn from them. Early and severe steps must be taken to identify the causes of this outbreak and halt its transmission before things burst out of control.
CONCLUSIONS
The recent rapid spread and emergence of monkeypox outside Africa is a cause of global concern. Many questions remain unanswered and under intense scrutiny by clinical and basic research and science community. Nevertheless, this disease must be adequately contained in multiple aspects, prioritize high-risk populations, and increase the investment in control, research and development in endemic African countries and those affected since 2022. In addition, education and prevention, mainly direct to vulnerable population like MSM are critical under the current circumstances of considering this an infection that can be transmitted during the personal and sexual contact, and other contagious routes.
Funding
There was no funding for this study.
Declaration of competing interest
The authors have no conflict of interest.
REFERENCES
[1] Cho CT, Wenner HA. Monkeypox virus. Bacteriol Rev. 1973; 37, 1-18.
[2] Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972; 46, 593-7.
[3] Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox - Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019; 78, 78-84.
[4] Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox - A potential threat? A systematic review. PLoS Negl Trop Dis. 2022; 16, e0010141.
[5] Sklenovská N, Van Ranst M. Emergence of monkeypox as the Most Important Orthopoxvirus Infection in Humans. Front Public Health. 2018; 6, 241.
[6] CDC. Multistate outbreak of monkeypox--Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003; 52, 537-40.
[7] Yong SEF, Ng OT, Ho ZJM, et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020; 26, 1826-30.
[8] Hobson G, Adamson J, Adler H, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021; 26. https://doi.org/10.2807/1560-7917.ES.2021.26.32.2100745.
[9] Rao AK, Schulte J, Chen T-H, et al. monkeypox in a Traveler Returning from Nigeria - Dallas, Texas, July 2021. MMWR Morb Mortal Wkly Rep. 2022; 71, 509-16.
[10] Costello V, Sowash M, Gaur A, et al. Imported monkeypox from International Traveler, Maryland, USA, 2021. Emerg Infect Dis. 2022; 28, 1002-5.
[11] Kabuga AI, El Zowalaty ME. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J Med Virol. 2019; 91: 533-40.
[12] Ruiz SI, Zumbrun EE, Nalca A. Chapter 38 - Animal Models of Human Viral Diseases. In: Conn PM, ed. Animal Models for the Study of Human Disease. Boston: Academic Press. 2013; 927-970.
[13] Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008; 225, 96-113.
[14] Kopecki D. Monkeypox outbreak is primarily spreading through sex, WHO officials say 2022. https://www.cnbc.com/2022/05/23/monkeypox-outbreak-is-primarily-spreading-through-sex-who-officials-say.html (accessed May 24, 2022).
[15] Diaz JH. The disease ecology, epidemiology, clinical manifestations, management, prevention, and control of increasing human infections with animal orthopoxviruses. Wilderness Environ Med. 2021; 32, 528-36.
[16] Kalthan E, Tenguere J, Ndjapou SG, et al. Investigation of an outbreak of monkeypox in an area occupied by armed groups, Central African Republic. Médecine Mal Infect. 2018; 48, 263-8.
[17] Yinka-Ogunleye A, Aruna O, Ogoina D, Aworabhi N, Eteng W, Badaru S, et al. Reemergence of Human monkeypox in Nigeria, 2017. Emerg Infect Dis. 2018; 24, 1149-51.
[18] Peter OJ, Kumar S, Kumari N, et al. Transmission dynamics of monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2021. https://doi.org/10.1007/s40808-021-01313-2.
[19] McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014; 58, 260-7.
[20] Petersen BW, Kabamba J, McCollum AM, et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antiviral Res. 2019; 162, 171-7.
[21] Graef S, Kurth A, Auw-Haedrich C, et al. Clinicopathological Findings in Persistent Corneal Cowpox Infection. JAMA Ophthalmol. 2013; 131, 1089.
[22] Goff AJ, Chapman J, Foster C, et al. A Novel Respiratory Model of Infection with monkeypox Virus in Cynomolgus Macaques. J Virol. 2011; 85, 4898-909.
[23] Nalca A, Livingston VA, Garza NL, et al. Experimental infection of Cynomolgus Macaques (Macaca fascicularis) with aerosolised monkeypox virus. PLoS One. 2010; 5, e12880. https://doi.org/10.1371/journal.pone.0012880.
[24] CDC. Monkeypox - Case Definition 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html (accessed May 26, 2022).
[25] Kulesh DA, Loveless BM, Norwood D, et al. Monkeypox virus detection in rodents using real-time 3’-minor groove binder TaqMan® assays on the Roche Light Cycler. Lab Investig. 2004; 84, 1200-8.
[26] Reynolds M, McCollum A, Nguete B, et al. Improving the Care and Treatment of monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017; 9, 380.
[27] Grosenbach DW, Honeychurch K, Rose EA, et al. Oral Tecovirimat for the Treatment of Smallpox. N Engl J Med. 2018; 379, 44-53.
[28] Chittick G, Morrison M, Brundage T, Nichols WG. Short-term clinical safety profile of brincidofovir: A favorable benefit–risk proposition in the treatment of smallpox. Antiviral Res. 2017; 143, 269-77.
[29] Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022; S1473-3099(22)00228-6.
[30] Fine PEM, Jezek Z, Grab B, Dixon H. The Transmission Potential of monkeypox Virus in Human Populations. Int J Epidemiol. 1988; 17, 643-50.
[31] WHO. Multicountry monkeypox outbreak in non-endemic countries 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed May 25, 2022).
[32] Weinstein RA, Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005; 41, 1765-71.
[32] CDC. Chart 1: Acute, Generalized Vesicular or Pustular Rash Illness Protocol 2022. https://www.cdc.gov/smallpox/lab-personnel/laboratory-procedures/chart1.html (accessed May 26, 2022).
[34] Falcinelli SD, Chertow DS, Kindrachuk J. Integration of global analyses of host molecular responses with clinical data to evaluate pathogenesis and advance therapies for emerging and reemerging viral infections. ACS Infect Dis. 2016; 2, 787-799.
[35] Zahra. MMF. Monkeypox, Treasure Island (FL): StatPearls; n.d.
[36] CDC. Monkeypox-Treatment 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html (accessed May 26, 2022).
[37] NewsWeek. How deadly is monkeypox? Virus fatality rate compared to COVID 2022. https://www.newsweek.com/how-deadly-monkeypox-virus-fatality-rate-compared-covid-1709269 (accessed May 28, 2022).
[38] Monkeypox n.d. https://www.who.int/news-room/fact-sheets/detail/monkeypox%0A (accessed May 26, 2022).
[39] Lai C-C, Wang C-Y, Hsueh P-R. Coinfections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020; 53, 505-12.
[40] Aghbash PS, Eslami N, Shirvaliloo M, Baghi HB. Viral coinfections in COVID-19. J Med Virol. 2021; 93, 5310-22.
[41] Fuller T, Thomassen HA, Mulembakani PM, et al. Using Remote Sensing to Map the Risk of Human monkeypox Virus in the Congo Basin. Ecohealth. 2011; 8, 14-25.
[42] Guagliardo SAJ, Doshi RH, Reynolds MG, et al. Do monkeypox Exposures Vary by Ethnicity? Comparison of Aka and Bantu Suspected monkeypox Cases. Am J Trop Med Hyg. 2020; 102, 202-5.
[43] Jezek Z, Fenner F. Human monkeypox. Karger. 1988. https://doi.org/10.1159/isbn.978-3-318-04039-5.
[44] Jezek Z, Grab B, Szczeniowski M V, Paluku KM, Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988; 66, 465-70.
[45] Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci. USA. 2010; 107, 16262-7.
[46] (ICTV) ICoToV. Virus Taxonomy 2020. http://www.ictv.org
[47] Hutin YJ, Williams RJ, Malfait P, et al. outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001; 7, 434.
[48] Doty JB, Malekani JM, Kalemba LN, et al. Assessing monkeypox virus prevalence in small mammals at the human–animal interface in the Democratic Republic of the Congo. Viruses. 2017; 9, 283.
[49] Nguyen P-Y, Ajisegiri WS, Costantino V. Reemergence of human monkeypox and declining population immunity in the context of urbanisation, Nigeria, 2017-2020. Emerg Infect Dis. 2021; 27, 1007.
[50] Simpson K, Heymann D, Brown CS, et al. Human monkeypox - After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020; 38, 5077-81.
[51] Kugelman JR, Johnston SC, Mulembakani PM, et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014; 20, 232.
[52] Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016; 1.
[53] Nigeria CDC. An update of monkeypox outbreak in Nigeria 2018.
[54] PHAC. Update on monkeypox in Canada - May 25, 2022.
[55] Suster D, Michal M, Huang H, et al. Myxoinflammatory fibroblastic sarcoma: an immunohistochemical and molecular genetic study of 73 cases. Mod Pathol. 2020; 33, 2520-33.
[56] Kozlov M. Monkeypox goes global: why scientists are on alert. Nature. 2022. https://doi.org/10.1038/d41586-022-01421-8.
[57] Hutson CL, Nakazawa YJ, Self J, et al. Laboratory Investigations of African pouched rats (Cricetomys gambianus) as a potential reservoir host species for monkeypox virus. PLoS Negl Trop Dis. 2015; 9, 1-20.
[58] Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013; 8, 129.
[59] Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986; 1, 98-99.
[60] Anonymous. Monkeypox outbreak questions intensify as cases soar. 2022. https://www.science.org/content/article/monkeypox-outbreak-questions-intensify-cases-soar
[61] Bass J, Tack DM, McCollum AM, et al. Enhancing Health Care Worker Ability to Detect and Care for Patients with monkeypox, Democratic Republic of the Congo. Int Health. 2013; 5, 237.
[62] ECDC. Monkeypox cases reported in UK and Portugal. 2022. https://www.ecdc.europa.eu/en/news-events/monkeypox-cases-reported-uk-and-portugal
[63] CDC. Infection Prevention and Control of monkeypox in Healthcare Settings. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
[64] Magnus Pv, Andersen EK, Petersen KB, Birch-Andersen A. A Pox-like disease in Cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959; 46, 156-76.
[65] Bonilla-Aldana DK, Rodriguez-Morales AJ. Is monkeypox another reemerging viral zoonosis with many animal hosts yet to be defined? Vet Q. 2022; 1-5. doi: 10.1080/01652176.2022.2088881.
[66] Rodríguez-Morales AJ, Ortiz-Martínez Y, Bonilla-Aldana DK. What has been researched about monkeypox? A bibliometric analysis of an old zoonotic virus causing global concern. New Microbes New Infect. 2022; 100993.
[67] Cheng K, Guo Q, Zhou Y, Wu H. Concern over monkeypox outbreak: What can we learn from the top 100 highly cited articles in monkeypox research? Travel Med Infect Dis. 2022; 102371.
[68] Cheng K, Zhou Y, Wu H. Bibliometric analysis of global research trends on monkeypox: Are we ready to face this challenge? J Med Virol. 2022. doi: 10.1002/jmv.27892.
[69] Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013; 8, 129-57.
[70] Bleyer JG. Ueber Auftreten von Variola unter Affen der Genera Mycetes und Cebus bei Vordringen einer Pockenepidemie im Urwaldgebiete an den Nebenflüssen des Alto Uruguay in Südbrasilien. Muench med Wochenschr. 1922; 69, 1009-10.
[71] León-Figueroa DA, Bonilla-Aldana DK, Pachar M, Romaní L, et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med Infect Dis. 2022; 49, 102362.
[72] Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004; 350 (4), 342-350.
[73] Farahat RA, Abdelaal A, Shah J, et al. Monkeypox outbreaks during COVID-19 pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob. 2022; 21. https://doi.org/10.1186/s12941-022-00518-2
[74] Haider N, Guitian J, Simons D, et al. Increased outbreaks of monkeypox highlight gaps in actual disease burden in Sub-Saharan Africa and in animal reservoirs. Int J Infect Dis. 2022; 122: 107-11.
[75] Radonić A, Metzger S, Dabrowski PW, et al. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerg Infect Dis. 2014; 20 (6), 1009-11.
[76] Doty JB, Malekani JM, Kalemba LN, et al. Assessing Monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses. 2017; 9 (10), 283.
[77] Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020; 12 (11), 1257.
[78] Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022; 27 (22). doi: 10.2807/1560-7917. ES.2022.27.22.2200421.
[79] Eltvedt AK, Christiansen M, Poulsen A. A Case Report of Monkeypox in a 4-Year-Old boy from the DR Congo: challenges of diagnosis and management. Case Rep Pediatr. 2020; 2020, 8572596.
[80] Bížová B, Veselý D, Trojánek M, Rob F. Coinfection of syphilis and monkeypox in HIV positive man in Prague, Czech Republic. Travel Med Infect Dis. 2022; 49, 102368.
[81] Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolised monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis). Lab Invest. 2001; 81 (12), 1581-600.
[82] Müller G, Meyer A, Gras F, Emmerich P, Kolakowski T, Esposito JJ. Monkeypox virus in liver and spleen of child in Gabon. Lancet. 1988; 1 (8588), 769-70.
[83] Rodriguez-Morales AJ. Monkeypox and the importance of cutaneous manifestations for disease suspicion. Microbes Infect Chemother. 2022; 2, e1450.
[84] Vivancos R, Anderson C, Blomquist P, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022; 27(22). doi: 10.2807/1560-7917.ES.2022.27.22.2200422.
[85] Hammerschlag Y, MacLeod G, Papadakis G, et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 2022; 27 (22). doi: 10.2807/1560-7917.ES.2022.27.22.2200411.
[86] Perez Duque M, Ribeiro S, Martins JV, et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022; 27 (22). doi: 10.2807/1560-7917. ES.2022.27.22.2200424.
[87] Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005; 41 (12), 1742-51.
[88] WHO. Clinical management and infection prevention and control for monkeypox: Interim rapid response guidance, Jun 10, 2022. https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1
[89] Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (Smallpox and Monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to Orthopoxviruses: recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022; 71 (22), 734-42.
[90] Patrocinio-Jesus R, and Peruzzu F. Monkeypox Genital Lesions. N Engl J Med. 2022. doi: 10.1056/NEJMicm2206893.
[91] Tumewu J, Wardiana M, Ervianty E, et al. An adult patient with suspected of monkeypox infection differential diagnosed to chickenpox. Infect Dis Rep. 2020; 12 (Suppl. 1), 8724. doi: 10.4081/idr.2020.8724.
[92] Meyer H, Perrichot M, Stemmler M, et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002; 40 (8), 2919-21.
[93] Ogoina D, Iroezindu M, James HI, et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin Infect Dis. 2020; 71 (8), e210-e214. doi: 10.1093/cid/ciaa143.
[94] Bhunu CP, Mushayabasa S, Hyman JM. Modelling HIV/AIDS and monkeypox co-infection. Applied Mathematics and Computation. 2012; 218, 9504-18.
[95] Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019; 19 (8), 872-9.
[96] Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998; 54 (3), 693-702.
[97] BHIVA. BHIVA rapid statement on monkeypox virus. https://www.bhiva.org/BHIVA-rapid-statement-on-monkeypox-virus
[98] BHIVA. BHIVA Vaccine Guidelines 2015 https://www.bhiva.org/file/NriBJHDVKGwzZ/2015-Vaccination-Guidelines.pdf
[99] Rodriguez-Morales AJ, González-Leal N, Montes-Montoya MC, et al. Cutaneous Larva Migrans. Curr Trop Med Rep. 2021; 8, 190-203.
[100] Virological. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak, May 2022 (confirmed case in Portugal). https://virological.org/t/first-draft-genome-sequence-of-monkeypox-virus-associated-with-the-suspected-multi-country-outbreak-may-2022-confirmed-case-in-portugal/799
[101] Virological. Belgian case of Monkeypox virus linked to outbreak in Portugal. https://virological.org/t/belgian-case-of-monkeypox-virus-linked-to-outbreak-in-portugal/801
[102] Virological. Multi-country outbreak of Monkeypox virus: genetic divergence and first signs of microevolution. https://virological.org/t/multi-country-outbreak-of-monkeypox-virus-genetic-divergence-and-first-signs-of-microevolution/806
[103] Virological. Update to observations about putative APOBEC3 deaminase editing in the light of new genomes from USA. https://virological.org/t/update-to-observations-about-putative-apobec3-deaminase-editing-in-the-light-of-new-genomes-from-usa/847
[104] Virological. First monkeypox virus genome sequence from Brazil. https://virological.org/t/first-monkeypox-virus-genome-sequence-from-brazil/850
[105] Virological. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. https://virological.org/t/urgent-need-for-a-non-discriminatory-and-non-stigmatizing-nomenclature-for-monkeypox-virus/853
[106] Basgoz N, Brown CM, Smole SC, et al. Case 24-2022: A 31-Year-Old Man with Perianal and Penile Ulcers, Rectal Pain, and Rash. N Engl J Med. 2022. doi: 10.1056/NEJMcpc2201244.
[107] Grant R, Nguyen LL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020; 98 (9), 638-40.
[108] Alkhalil, A., Hammamieh, R., Hardick, J. et al. Gene expression profiling of monkeypox virus-infected cells reveals novel interfaces for host-virus interactions. Virol J. 2010; 7, 173.
[109] Lu Y and Zhang L. DNA-Sensing Antiviral Innate Immunity in Poxvirus Infection. Front. Immunol. 2020; 11, 1637.