Le Infezioni in Medicina, n. 3, 362-371, 2022
doi: 10.53854/liim-3003-5
REVIEWS
Pre-Exposure Prophylaxis for viral infections other than HIV
Vicente Soriano1, Ana Treviño1, Carmen de Mendoza2, Víctor Moreno-Torres2, Ilduara Pintos2, Pablo Barreiro1,3 and Octavio Corral1
1UNIR Health Sciences School & Medical Center, Pozuelo de Alarcón, Madrid, Spain;
2Puerta de Hierro Research Institute & University Hospital, Majadahonda, Madrid, Spain;
3Emergency Hospital Isabel Zendal, Madrid, Spain
Article received 5 July 2022, accepted 25 July 2022
Corresponding author
Vicente Soriano
E-mail: vicente.soriano@unir.net
SummaRY
The battle against human viral infections has historically relied on two medical strategies, namely vaccines to protect from contagion and antivirals to treat infected patients. In the absence of vaccines, antivirals have occasionally been used as peri-exposure prophylaxis, given either before (pre-exposure prophylaxis) or right after (post-exposure prophylaxis). In an unprecedented way, the use of antiretrovirals as chemoprophylaxis has triumphed in the HIV field. Indeed, oral antiretrovirals given either daily or at demand to HIV-uninfected individuals engaged in high-risk behaviors protect from contagion. More recently, the advent of long-acting formulations has allowed HIV protection following intramuscular injections every three months. Can we envision a similar prophylactic strategy for other human viral infections?
The advent of such ‘chemical vaccines’ would fill an unmet need when classical vaccines do not exist, cannot be recommended, immune responses are suboptimal, escape mutants emerge or immunity wanes. In this review, we discuss the opportunities for antiviral chemoprophylaxis for viral hepatitis B and C, retroviruses HTLV-1 and HIV-2, and respiratory viruses influenza and SARS-CoV-2, among others.
Keywords: antiviral drugs, prevention, prophylaxis, hepatitis C, hepatitis B, HTLV-1, SARS-CoV-2, monkeypox virus, HIV, long-acting antivirals.
INTRODUCTION
Since the advent of sulfamides and penicillin as first widely used antibiotics during World War II, viruses have steadily replaced bacteria as major infectious agents, particularly for globally spread microorganisms. Examples are pandemics caused by influenza, the human immunodeficiency virus (HIV), hepatitis B and C viruses (HBV and HCV), Dengue, Zika and nowadays SARS-CoV-2, the coronavirus responsible for the COVID-19 pandemic [1].
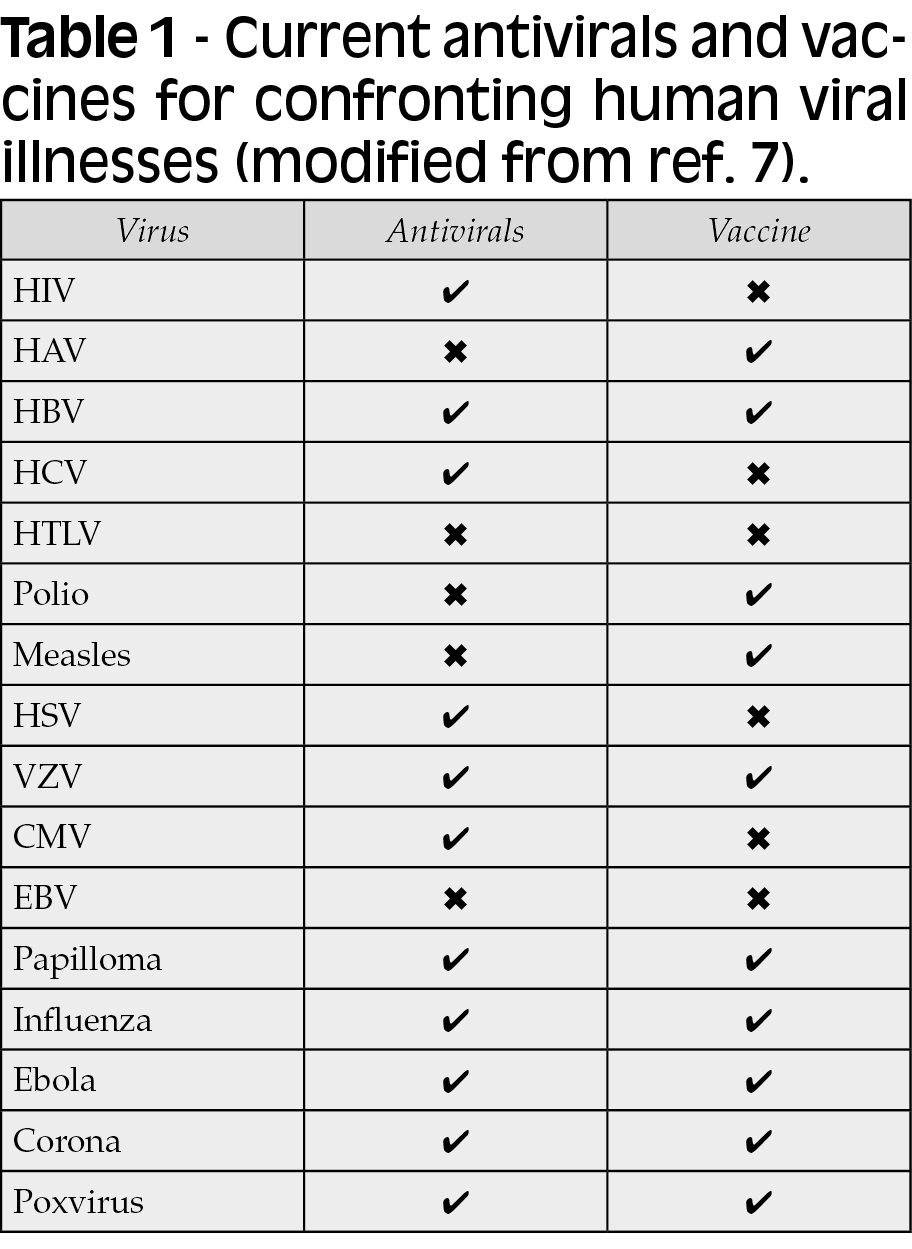
The battle against human viral infections has historically relied on two medical strategies, namely vaccines to protect from contagion and antivirals to treat infected patients. For different reasons, the development of effective vaccines and/or antivirals has been achieved for some but not all viruses (Table 1). Vaccines use immunogens that awake specific adaptive humoral and/or cellular immune responses in the host, preventing the acquisition of a given infectious agent. In contrast, antiviral drugs target either unique viral proteins or cellular molecules that are critical for viral replication. Antivirals that exclusively target viral enzymes benefit from having no expected effects on human proteins, and therefore less chances to produce adverse events [2].
In the absence of vaccines, antivirals have occasionally been used as peri-exposure prophylaxis, given either before (pre-exposure prophylaxis, PrEP) or right after (post-exposure prophylaxis). In an unprecedented way, the use of chemoprophylaxis has been fueled in the HIV field as protection from contagion [3]. Two major reasons account for this phenomenon. Firstly, HIV vaccines have remained elusive to date despite huge efforts. Secondly, the development of long-acting formulations of potent antiretrovirals has shown to be very effective, protecting uninfected individuals with high risk behaviors [4-6]. Intramuscular or subcutaneous shots given a few times per year or even once yearly might work as ‘chemovaccines’ [7-10]. Can we envision such approach to protect from other human viral infections?
The advent of such ‘chemical vaccines’ would fill an immediate need in providing protection when classical vaccines do not exist, cannot be recommended, immune responses are suboptimal, escape mutants emerge or immunity wanes (Table 2) [11]. In this review, we discuss the opportunities for antiviral chemoprophylaxis for viral hepatitis B and C, retroviruses HTLV-1 and HIV-2, and respiratory viruses influenza and SARS-CoV-2.

Hepatitis B
Roughly two billion people have been exposed to the hepatitis B virus (HBV) worldwide. Estimates for chronic hepatitis B patients are of 290 million people. The number of global deaths caused each year by HBV due to decompensated cirrhosis or liver cancer approach one million. These high numbers are paradoxical considering that effective protective HBV vaccines exist for more than 40 years. Suboptimal global HBV vaccine coverage largely explains it [12].
Antiviral agents to treat HBV have also been available for decades. Nowadays tenofovir and entecavir have replaced lamivudine as oral anti-HBV agent. Although viral suppression is achieved in most treated patients, HBV is not eradicated form carriers and therefore oral treatment is lifelong. Patients with chronic hepatitis B under long-term tenofovir experience a reduced risk of liver disease progression and hepatocellular carcinoma. In addition, viral suppression is associated to halted HBV transmission, a benefit known as ‘treatment as prevention’ [3].
The first observations about a protective effect of tenofovir on HBV acquisition come from studies conducted in HIV patients on antiretroviral therapy [13, 14]. Sexually active men having sex with men (MSM) that received tenofovir had a lower chance of HBV infection than those that did not receive the drug. Thereafter, this protective effect was confirmed in both HIV-infected individuals under tenofovir-based antiretroviral therapy and in HIV-uninfected persons at risk undergoing PrEP with tenofovir. Based on this finding, the increasing trend in HIV therapeutics for using mono or dual regimens sparing tenofovir is worrisome [15]. It might be associated with a rebound of incident HBV infections.
Although HBV vaccines are effective, individuals with immunosuppression may experience more frequently suboptimal responses and/or waning of antibodies following immunization [16]. Moreover, circulation of HBV vaccine escape mutants is occasionally responsible for paradoxical acute HBV infections in vaccinated individuals [17].
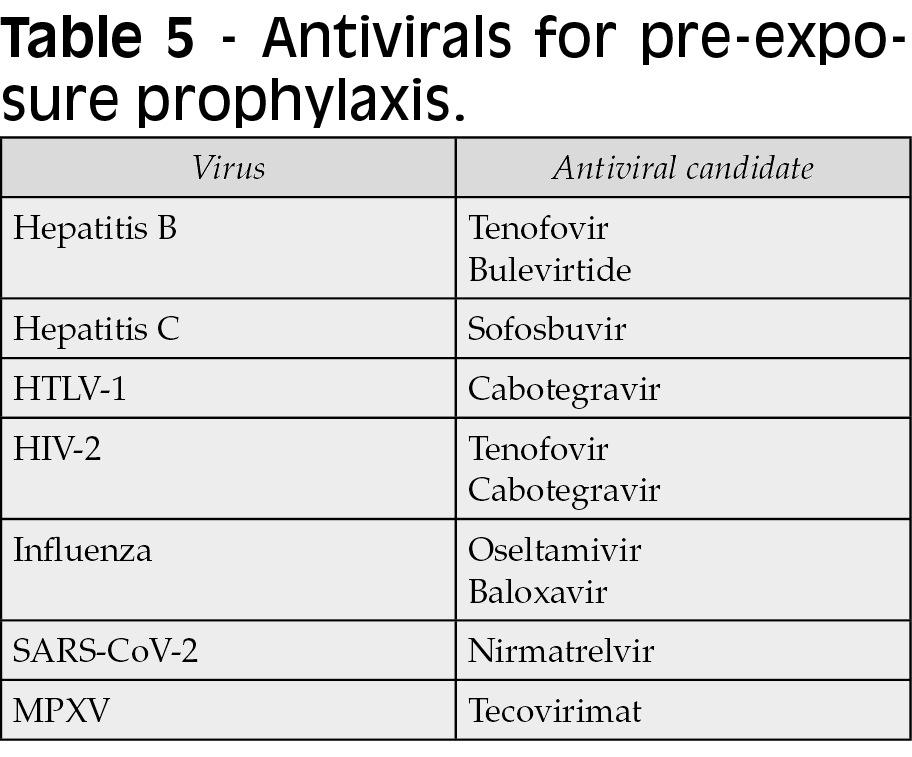
For all these considerations, the use of tenofovir as chemoprophylaxis might be discussed in certain populations to avert breakthrough HBV infections. We postulate that this could be the case for immunodeficient individuals engaged in high risk behaviors, including injection drug users (IDUs) that share needles, MSM and persons with multiple sexual partners (Table 3). The advent of long-acting formulations of tenofovir could facilitate this option [18]. An additional indirect benefit of using tenofovir chemoprophylaxis would derive from reducing the chances of hepatitis delta virus (HDV) acquisition, since this virus requires HBV for transmission [18, 19].

Hepatitis C
For long time, infection with the hepatitis C virus (HCV) has been one of the most frequent causes of decompensated cirrhosis and liver cancer worldwide and particularly in developed countries. The advent of new oral direct-acting antivirals during the last decade has transformed the HCV landscape [21]. Oral treatments generally given for three months can cure (eradicate) hepatitis C in most treated patients.
Global estimates for HCV viremic persons have declined from 70 million to 50 million as result of the widespread use of oral new HCV therapies. Successful plans at country level, such as in Egypt or Georgia, highlight this historical benefit Egypt has been the first country to eliminate hepatitis C, curing more than 4 million patients [22, 23].
Treatment failures to new oral anti-HCV agents are very rare and generally associated to poor drug adherence and/or selection of drug resistance. On the other hand, HCV re-infections may occur in persons with high risk behaviors since there is no protective HCV immunity [24]. However, anti-HCV re-treatment even with the same regimen that cured the first HCV episode, is generally successful again [25]. Thus, any consideration about prophylaxis with antivirals is less appealing for HCV than for hepatitis B or HIV, given that any course of HCV therapy generally leads to hepatitis C cure. However, in certain risk populations, such as IDUs sharing needles and MSM with multiple sex partners, repeated episodes of HCV re-infection (occasionally more than 6) have urged to reconsider this strategy [26]. Given that the clinical consequences of chronic hepatitis C are generally seen only after decades of infection, some authors advice against immediate anti-HCV treatment risk behaviors persist. On the other hand, being viremic means to be potential source of contagion for others [27, 28]. In this scenario, the consideration of sofosbuvir as prevention of new HCV infections in persons engaged in risky behaviors and/or in prisons could be defended (Figure 1). Sofosbuvir is an attractive agent for this objective, given its high potency, good safety profile, scarce drug interactions and high barrier to resistance. After becoming HCV-negative following a first course of therapy, sofosbuvir chemoprophylaxis could avert HCV re-infections. Furthermore, if long-acting forms of HCV drugs become available, this option could be even more appealing [29].
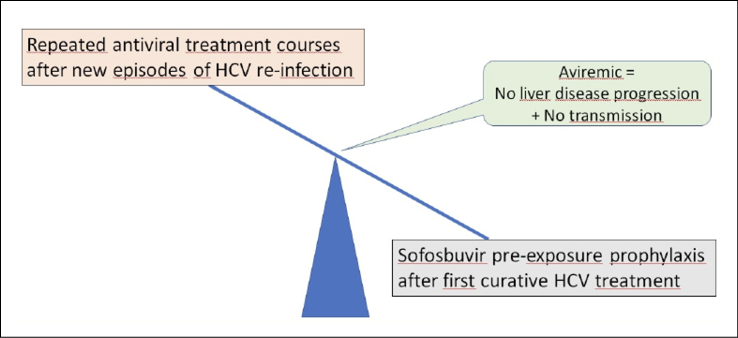
Figure 1 - Management of hepatitis C in persons engaged in high risk behaviors.
A different scenario for considering HCV chemoprophylaxis is represented by recipients of HCV+ organ transplants (kidney, liver, etc.). Although HCV donors were traditionally discharged, they are increasingly being accepted given the shortage of organs for transplantation and the fact that early administration of HCV antivirals precludes new onset hepatitis C in the recipient [30].
HTLV-1
Other retroviruses than HIV-1 may cause human diseases. HTLV-1 infects 15-20 million people worldwide and may produce two characteristic illnesses in 10% of carriers, namely a subacute myelopathy known as tropical spastic paraparesis (or HTLV-associated myelopathy) and adult T-cell lymphomas. The virus is transmitted throughout sexual contact, from infected mothers to newborns mostly through breastfeeding, and following parenteral exposure (transfusions, organ transplantation and injection drug use) [31].
Despite being present globally, hot spots of endemicity exist for HTLV-1 in Latin America, the Caribbean basin, West Africa, South of Japan, Northeast Iran, Australia and Romania [31]. Following large population migration flows, HTLV-1 is currently been reported in North America and the European Union, mostly among foreigners coming from endemic regions [32]. Moreover, new HTLV-1 diagnoses among natives are increasingly been reported in sexual partners [33]. Given that there is neither protective vaccine nor effective antivirals to treat HTLV-1 infection or its complications, early diagnosis has become an important tool. Only in this way, asymptomatic HTLV-1 carriers can be unveiled, start periodic clinical follow-up and be informed about the risks of transmission. In this regard, testing of sex partners and avoiding breastfeeding are very effective measures [34].
The incidence of sexually transmitted infections (STI) is on the rise during the last decade. The loss of fear to HIV contagion following the success of PrEP has largely contributed to it [35]. In response to this ‘compensatory effect’, current guidelines recommend STI testing before starting and periodically in all individuals on HIV PrEP. Besides classical STI agents, i.e. syphilis, gonorrhea, chlamydia and mycoplasma, testing for other less frequent etiologies is increasingly becoming cost-effective and informative in terms of public health. We and others support that this should be the case for HTLV-1; which should be added into the STI screening list [34-36].
Antiretrovirals used as HIV PreP include nucleos(t)ide reverse transcriptase inhibitors and integrase inhibitors. There is a lack of HTLV-1 seroprevalence studies among HIV PrEP-using groups, including MSM, IDUs, and female sex workers. These HTLV-1 serosurveys should be performed to recover any evidence in favor of a protective effect of antiretrovirals on HTLV-1 acquisition. This information would be particularly relevant in cabotegravir users, since this drug has shown potent antiretroviral activity in vitro against HTLV-1 [37, 38].
HIV-2
Around 1-2 million people are infected with HIV-2 worldwide. Endemic areas exist in West Africa, Brazil, India and Portugal. HIV-2 has also been described in Western countries with a large immigrant population from HIV-2 endemic regions. In this way, nearly 500 cases of HIV-2 have been reported in Spain [39].
HIV-2 may cause AIDS but slowly than HIV-1. Although many antiretrovirals used to treat HIV-1 are active against HIV-2, neither non-nucleoside reverse transcriptase inhibitors nor entry inhibitors are active. Moreover, some HIV-1 protease inhibitors are less effective against HIV-2. Finally, drug resistance mutations in HIV-2 depict unique profiles [40].
Both daily and on-demand antiretroviral regimens have been proven effective as PrEP in HIV-1, but scarce data exist about protection exerted on HIV-2. Overall, HIV-2 is less transmissible than HIV-1 following sexual contact [3, 41, 43]. It is noteworthy that tenofovir and integrase inhibitors used as HIV-1 PrEP may protect from HIV-2 infection. In this regard, long-acting formulations of cabotegravir might prevent from sexually transmitted HIV-2 in endemic regions or in persons with an HIV-2 sex partner. The use of other antiretroviral agents, however, could not prevent HIV-2 acquisition [43].
Influenza
Influenza viruses of types A and B attack 5-10% of adults and 20-30% of children, thereby causing millions of acute respiratory infections annually. A significant number of these episodes are associated with complications such as pneumonia and bacterial superinfections that require hospitalization and might lead to death [44]. Vaccination is the most effective way to prevent influenza. If vaccination is not available, e.g., upon emergence of a new variant or in vulnerable groups with diminished immune responses, prophylaxis and/or early treatment with antivirals represents an alternative option [45]. The timely prescription of antiviral drugs may shorten viral shedding, reduce symptoms, and diminish contagiousness.
There are currently three classes of antivirals for treatment and prophylaxis of influenza: neuraminidase inhibitors (oseltamivir and zanamivir), M2 ion channel inhibitors (adamantanes), and Cap-dependent endonuclease inhibitor baloxavir. The efficacy of adamantanes (amantadine and rimantadine) is limited since they are not effective against influenza B viruses and high rates of drug resistance in influenza A are seen since 2004 [46]. Treatment of influenza with neuraminidase inhibitors is recommended for patients at high risk for developing severe illness or infected with avian influenza viruses like A(H5N1).
As PrEP, inhaled zanamivir is approved for 28 days, whereas oral oseltamivir is approved for up to 6 weeks in the general population, and for 12 weeks in immunosuppressed individuals. Usually, the dosage of neuraminidase inhibitors used for prophylaxis is only half of that used for treatment. Interestingly, there is no cross-resistance between oseltamivir and zanamivir [46].
Baloxavir is an oral prodrug administered as a single dose for the treatment of acute uncomplicated influenza. Baloxavir blocks the viral RNA-dependent RNA polymerase. It has a broad spectrum coverage and represents an option for patients with infections caused by drug-resistant influenza viruses. However, itself depicts a low barrier to resistance. In susceptible individuals, baloxavir shortens the length of viral shedding by a mean of 2 days. When used as prophylaxis, baloxavir reduces the risk of developing influenza by 86% [46].
Influenza antivirals used as pre- and post-exposure prophylaxis represent an essential tool to stop outbreaks in institutions, such as hospitals or nursery homes. Oseltamivir remains the drug of choice for influenza treatment and prevention nowadays. Antiviral treatment of influenza should be restricted to severe illness in critically ill hospitalized patients and should be considered for in- and outpatients who are at high risk for severe disease. In special cases, Baloxavir may represent an attractive option with a single dose oral regimen.
SARS-CoV-2
The unprecedented COVID-19 pandemic has transformed and accelerated drug discovery and marketing paths. Three antivirals have been approved so far against SARS-CoV-2, known as remdesivir, molnupiravir and nirmatrelvir. Remdesivir is an adenosine prodrug given parenterally that exerts moderate antiviral activity as chain terminator during SARS-CoV-2 replication. Molnupiravir is an oral prodrug form of the cytidine nucleoside, which can be metabolized and incorporated into the viral RNA, leading to viral mutagenesis and error catastrophe. Nirmatrelvir is an oral specific SARS-CoV-2 protease inhibitor, which blocks viral replication by inhibitory competition. It exerts the greatest antiviral activity on SARS-CoV-2 [47].
In clinical trials, both oral nirmatrelvir and molnupiravir, when administered during the initial stages of SARS-CoV-2 infection, have shown a reduction in the risk of hospitalization or death in high-risk adult outpatients with mild to moderate symptoms [48]. Paxlovid® is more potent than the other two drugs. It is currently being tested in standard-risk patients early after the onset of the disease, assessing the time interval to sustained symptom relief. The drug is also being evaluated for administration as post-exposure prophylaxis, in individuals exposed to symptomatic patients with laboratory-confirmed SARS-CoV-2 infection.
Oral antivirals can strengthen the efforts to quell the health impacts of the COVID-19 pandemic, especially facing the continuous emergence of new SARS-CoV-2 variants, characterized by spike mutations and immune escape. Their role should be considered complementary to vaccination, which remains the key to tackling the COVID-19 pandemic.
Given that SARS-CoV-2 primarily infects the upper respiratory tract, there is much interest for exploring the performance of inhaled forms of antivirals [49]. Besides targeting the anatomic viral entry site, this route might reduce systemic side effects and concerns on drug interactions.
Monkeypox virus
Smallpox has been one of the most deadly human infections historically. It is caused by the variola virus that belongs to the poxvirus family. Vaccination begun two centuries ago and finally accomplished the objective of eradicating the disease. The last case was reported in Somalia in 1977. The WHO declared smallpox as completely eliminated in 1980. Since then, vaccination was discontinued [50].
Other poxviruses, however, such as molluscum contagiosum, cowpox and monkeypox, remain in the natural environment and can infect both animals and humans. Poxviruses causing animal illnesses can occasionally jump to humans and produce outbreaks. In year 2003 there was an outbreak in the United States of monkey poxvirus (MPXV) that affected more than 40 persons [51]. All sick patients were exposed to pets that had been growth in a farm where other small animals had recently arrived from West Africa, where MPXV is endemic.
Poxviruses are very large particles (up to 400 nm), even visible using the optic microscope. They contain a single double stranded large DNA ranging from 130 to 375 Kb. In contrast with retroviruses or hepatitis B, replication of poxviruses only takes place within the cytosol of infected cells.
In early May 2022, an outbreak of MPXV begun in Europe. Nearly 10,000 cases were confirmed during the next 3 months with a wide international distribution. Interestingly, most cases occurred among MSM, younger than 45 years-old, had genital and anal skin lesions, and self-limited within 2-3 weeks with very few deaths [52]. This MPXV epidemic form contrast with the endemic form in West and Central Africa, first described in the Democratic Republic of Congo by 1970. African cases are seen occasionally as a zoonosis in persons with close contact with infected rodents, the natural reservoir. The mortality rate ranges from 3 to 10% [53].
Globally all individuals older than 45 years-old are vaccinated against smallpox and exhibit cross-protection against MPXV infection. In this regard, the safer smallpox vaccine registered by Bavarian Nordic, a Danish biotech company that uses a non-replicative virus, largely protects from MPXV contagion. In addition, there is an oral antiviral drug that is effective against MPXV, called tecovirimat. It is a specific inhibitor of the viral protein VP37, which is critical for viral replication. In adults, 600 mg twice daily are recommended for 14 days [54].
More severe forms of MPXV infection, including dissemination and multiorgan damage have been described in three populations: children, pregnant women and immunocompromised patients [55]. In these groups, PrEP with tecovirimat could be considered in the presence of close contacts with MPXV infection, i.e., sexual partners or relatives.
Caveats and concerns using PrEP for non-HIV infections
The use of antivirals as prevention rather than treatment of viral infections is steadily gaining support to prevent serious illnesses. Mimicking the protective effect of vaccines, PrEP with antivirals will reduce the chances of acquiring potentially life-threatening infections. The advent of long-acting formulations may further encourage PrEP use with antivirals for infections other than HIV-1. However, enthusiasm unabated, several concerns require attention (Table 4). There are at least four major considerations.

Firstly, selection of drug resistance may compromise the sustained benefit of antivirals, often used as monotherapies. For this reason, ideally drugs with high barrier to resistance must be considered as preferred candidates for PrEP. Suboptimal drug exposure in the presence of residual virus replication is the major mechanism for selection of drug resistance. This is the case for individuals that did not attend appointments on time for drug administration, even when recommended every 2-3 months. Lessons from the HIV field using long-acting cabotegravir may enlighten this caveat [56].
Secondly, drug interactions must be taken into consideration. Drug exposure cannot be interrupted abruptly in persons on long-acting antivirals. Both patients and their doctors must be aware of the potential risk of newly prescribed meds for any given medical condition. A careful assessment of potential drug interactions should always precede the introduction of new therapies. Safer drug alternatives should be chosen when potential drug interactions are expected.
Thirdly, the long-term side effects of antivirals used as extended released formulations must be monitored. Toxicities might appear in only a subset of treated patients and be misrecognized if there is no close follow-up. Particular attention should be paid for establishing the safety and efficacy of very long-acting products in women of reproductive age, those who are pregnant, or are breastfeeding.
Finally, the cost of medications is an important caveat. Discussions are softened when drugs are needed as treatment and alternative options are scarce. However, prescription of drugs as prevention is always hesitant, as questions arise about what can be made to push other preventive measures. This has occurred with HIV risk behaviors and will occur with respect to risk exposures to other viruses. It seems reasonable to make efforts for providing proper information and education, and support safer life style changes in advance [3, 35, 57].
PrEP with antivirals in the era of precision medicine
Precision medicine is moving the medical paradigm from producing a diagnosis for any disease based on clinical symptoms/signs and complementary exams to predict the risk of illnesses in healthy persons based on individual genomics, biomarkers and imaging [58]. In the field of infectious diseases, precision medicine has become mostly personalized addressing the prevention of human infections. Tailoring the most convenient intervention for each individual may maximize the chances of success either for treatment or prevention [59].
The HIV field has been upfront in this new medical paradigm proving the efficacy of antiretrovirals as PrEP. The advent of long-acting antiretroviral formulations to be used as PrEP has proven very effective using intramuscular or subcutaneous injections just once or a few times yearly. Are we ready to consider a similar approach confronting other common human viral infections? [60-62]. We have identified distinct patient populations and specific clinical settings in which there is room for considering chemoprophylaxis for viral hepatitis B or C, retroviruses HTLV-1 or HIV-2, or respiratory viruses such as influenza or SARS-CoV-2.
References
[1] Morens D., Fauci A. Emerging pandemic diseases: how we got to COVID-19. Cell. 2020; 182:1077-1092.
[2] De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016; 29, 695-747.
[3] Fernández-Montero J.V., Barreiro P., Del Romero J., Soriano V. Antiretroviral drugs for pre-exposure prophylaxis of HIV infection. AIDS Rev. 2012; 14, 54-61.
[4] Landovitz R., Donnell D., Clement M., et al. HPTN 083 Study Team. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021; 385, 595-608.
[5] Thoueille P., Choong E., Cavassini M., Buclin T., Decosterd L. Long-acting antiretrovirals: a new era for the management and prevention of HIV infection. J Antimicrob Chemother. 2022; 77, 290-302.
[6] Cobb D., Smith N., Edagwa B., McMillan E. Long-acting approaches for delivery of antiretroviral drugs for prevention and treatment of HIV: a review of recent research. Expert Opin Drug Deliv. 2020; 17, 1227-1238.
[7] Kulkarni T., Bade A., Sillman B., et al. A year-long extended release nanoformulated cabotegravir prodrug. Nat Mater. 2020; 19, 910-920.
[8] Soriano V., Barreiro P., de Mendoza C. Long-acting antiretroviral therapy. Nat Mater. 2020; 19, 826-827.
[9] Gautam N., McMillan J.M., Kumar D., et al. Lipophilic nanocrystal prodrug-release defines the extended pharmacokinetic profiles of a year-long cabotegravir. Nat Commun. 2021; 12, 3453.
[10] Marrazzo J. Lenacapavir for HIV-1 - potential promise of a long-acting antiretroviral drug. N Engl J Med. 2022; 386: 1848-1849.
[11] Soriano V., Edagwa B., de Mendoza C., et al. Ultra-Long-acting antivirals as chemical vaccines to prevent viral diseases. Future Microbiol (in press).
[12] Ward J., Hinman A. What is needed to eliminate hepatitis B virus and hepatitis C virus as global health threats. Gastroenterology. 2019; 156, 297-310.
[13] Gatanaga H., Hayashida T., Tanuma J., Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis. 2013; 56, 1812181-9.
[14] Heuft M., Houba S., van den Berk G., et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS. 2014; 28, 999-1005.
[15] Bollinger R., Thio C., Sulkowski M., McKenzie-White J., Thomas D., Flexner C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. Lancet HIV. 2020;7:e443-e448.
[16] Kim Y, Loucks J, Shah M. Efficacy of hepatitis B vaccine in adults with chronic liver disease. J Pharm Pract. 2022 Feb 24, 8971900221078742.
[17] Besombes J., Souala F., Bouguen G., et al. Acute hepatitis B virus infection despite vaccination in a patient treated by infliximab: a case report. BMC Gastroenterol. 2022; 22, 322.
[18] Cobb D., Smith N., Deodhar S., et al. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nat Commun. 2021; 12, 5458.
[19] Soriano V., Alvarez C., Edagwa B., de Mendoza C., Montoya N., Treviño A., Gendelman H. Ultra-long-acting (XLA) antivirals for chronic viral hepatitis. Int J Infect Dis. 2022; 114, 45-50.
[20] Soriano V., Mendoza C., Barreiro P., Treviño A., Corral O. Envisioning a hepatitis delta cure with new antivirals. Future Microbiol. 2021; 16: 927-930.
[21] Ramos-Rincon J.M., Pinargote-Celorio H., de Mendoza C., et al. Liver cancer and hepatic decompensation events in patients hospitalized with viral hepatitis in Spain. Hepatol Int. 2022 (in press).
[22] Waked I., Esmat G., Elsharkawy A., et al. Screening and treatment program to eliminate hepatitis C in Egypt. N Engl J Med. 2020; 382, 1166-1174.
[23] Tsertsvadze T., Gamkrelidze A., Nasrullah M., et al. Treatment outcomes of patients with chronic hepatitis C receiving sofosbuvir-based combination therapy within national hepatitis C elimination program in the country of Georgia. BMC Infect Dis. 2020; 20, 30.
[24] Johannesson J., Fridriksdottir R., Löve T., et al. High rate of HCV reinfection among recently injecting drug users: results from the TRAP Hep C program - a prospective nationwide, population-based study. Clin Infect Dis. (in press).
[25] Girometti N., Devitt E., Phillips J., Nelson M., Whitlock G. High rates of unprotected anal sex and use of generic direct-acting antivirals in a cohort of MSM with acute HCV infection. J Viral Hepat. 2019; 26, 627-634.
[26] Ingiliz P., Martin T.C., Rodger A., et al. NEAT study group. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J Hepatol. 2017;66:282-287.
[27] Fernández-Montero J.V., Aspinall E., Burns J. Treatment as prevention: should hepatitis C learn the lessons from HIV? AIDS Rev. 2017; 19, 212-218.
[28] Spaulding A., Anderson E., Khan M., Taborda-Vidarte C., Phillips J. HIV and HCV in U.S. prisons and jails: the correctional facility as a bellwether over time for the community’s infections. AIDS Rev. 2017; 19, 134-147.
[29] Verma M., Chu J., Salama J., et al. Development of a long-acting direct-acting antiviral system for hepatitis C virus treatment in swine. Proc Natl Acad Sci USA. 2020;117: 11987-11994.
[30] Chen R., Li D., Zhang M., Yuan X. Sofosbuvir/velpatasvir prophylaxis for 12 weeks in HCV-negative recipients receiving kidney transplantation from HCV-positive donors. Ann Transplant. 2021; 26, e933313.
[31] Legrand N., McGregor S., Bull R., et al. Clinical and public health implications of HTLV-1 infection. Clin Microbiol Rev. 2022; 35, e0007821.
[32] Ramos J.M., de Mendoza C., Aguilera A., et al. Spanish HTLV Network. Hospital admissions in individuals with HTLV-1 infection in Spain. AIDS. 2020; 34, 1019-1027.
[33] de Mendoza C., Caballero E., Aguilera A., et al. Spanish HTLV Network. HTLV-1 infection and disease in Spain. AIDS. 2017; 31, 1653-1663.
[34] De Mendoza C., Pérez L., Fernández-Ruiz M., et al. Late presentation of HTLV-1 infection in Spain reflects suboptimal testing strategies. Int J Infect Dis (in press).
[35] Soriano V., del Romero J. Rebound in sexually transmitted infections following the success of antiretrovirals for HIV/AIDS. AIDS Rev. 2018; 20, 187-204.
[36] Caswell R., Nall P., Boothby M., Taylor G. Rapid onset and progression of myelopathy following an STI: a case for screening? Sex Transm Infect. 2019; 95, 244-245.
[37] Bradshaw D., Taylor G. HTLV-1 transmission and HIV pre-exposure prophylaxis: a scoping review. Front Med (Lausanne). 2022; 9, 881547.
[38] Schneiderman B., Barski M., Maertens G. Cabotegravir, the long-acting integrase strand transfer inhibitor, potently inhibits HTLV-1 transmission in vitro. Front Med (Lausanne). 2022; 9, 889621.
[39] De Mendoza C., Lozano A.B., Caballero E., Cabezas T., Ramos J.M., Soriano V. Antiretroviral therapy for HIV-2 infection in non-endemic regions. AIDS Rev. 2020; 22, 44-56.
[40] Tzou P., Descamps D., Rhee S., et al. Expanded spectrum of antiretroviral-selected mutations in HIV-2. J Infect Dis. 2020; 221: 1962-1972.
[41] Molina J.M., Capitant C., Spire B., et al. ANRS IPERGAY Study Group. On-demand pre-exposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015; 373, 2237-2246.
[42] Gottlieb G., Hawes S., Agne H., et al. Lower levels of HIV RNA in semen in HIV-2 compared with HIV-1 infection: implications for differences in transmission. AIDS. 2006; 20, 895-900.
[43] Ceia F., Silva-Pinto A., Carvalho A., et al. HIV-2 superinfection in a patient receiving antiretroviral therapy with longstanding HIV-1 viral load suppression. Open Forum Infect Dis. 2019; 6, ofz063.
[44] Duwe S., Schmidt B., Gärtner B., et al. Prophylaxis and treatment of influenza: options, antiviral susceptibility, and existing recommendations. GMS Infect Dis. 2021; 9, Doc02.
[45] Gärtner B., Avery R. Respiratory viral infections in solid organ transplant recipients – New insights from multicenter data. Am J Transplant. 2020; 21, 1685-1686.
[46] Govorkova E., Takashita E., Daniels R., et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018-2020. Antiviral Res. 2022; 200, 105281.
[47] Soriano V., de Mendoza C., Edagwa B., Treviño A., Barreiro P., Fernández-Montero J.V., Gendelman H. Oral antivirals for the prevention and treatment of SARS-CoV-2 infection. AIDS Rev. 2022; 24, 41-49.
[48] Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, non-hospitalized adults with Covid-19. N Engl J Med. 2022; 386, 1397-408.
[49] Sahin G., Akbal-Dagistan O., Culha M., et al. Antivirals and the potential benefits of orally inhaled drug administration in COVID-19 treatment. J Pharm Sci. 2022 Jun 9, S0022-3549(22)00248-9.
[50] Moore Z., Seward J., Lane J. Smallpox. Lancet. 2006; 367, 425-435.
[51] Reed K, Melski J, Graham M, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004; 350, 342-350.
[52] Soriano V., Corral O. International outbreak of monkeypox in men having sex with men. AIDS Rev. 2022 (in press).
[53] Adler H., Gould S., Hine P., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022 May 24, S1473-3099(22)00228-6.
[54] Rizk J., Lippi G., Henry B., Forthal D., Rizk Y. Prevention and treatment of monkeypox. Drugs. (in press).
[55] Wendt R., Tittelbach J., Schrick L., et al. Generalized cowpox virus infection in an immunosuppressed patient. Int J Infect Dis. 2021; 106, 276-278.
[56] Eshleman S., Fogel J., Piwowar-Manning E., et al. Characterization of HIV infections in women who received injectable cabotegravir or tenofovir disoproxil fumarate/emtricitabine for HIV prevention: HPTN 084. J Infect Dis. 2022; 225, 1741-1749.
[57] Soriano V., Gallego L. Viral hepatitis: Treating hepatitis C in injection drug users. Nat Rev Gastroenterol Hepatol. 2013; 10, 568-569.
[58] Hou Y.-C., Yu H., Martin R., et al. Precision medicine integrating whole-genome sequencing, comprehensive metabolomics and advanced imaging. Proc Natl Acad Sci USA. 2020; 17, 3053-3062.
[59] Mogensen T. Genetic susceptibility to viral disease in humans. Clin Microbiol Infect. 2022 (in press).
[60] Shi Y., Lu A., Wang X., Belhadj Z., Wang J., Zhang Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm Sin B. 2021; 11, 2396-415.
[61] Markovic M, Deodhar S, Machhi J, et al. Prodrug therapies for infectious and neurodegenerative diseases. Pharmaceutics. 2022; 14, 518.
[62] Chu J., Traverso G. Foundations of gastrointestinal-based drug delivery and future developments. Nat Rev Gastroenterol Hepatol. 2022; 19, 219-2138.