Le Infezioni in Medicina, n. 3, 353-361, 2022
doi: 10.53854/liim-3003-4
REVIEWS
Acute hepatitis (Non Hepa A-E) of unknown origin among pediatrics
Nasim Asadi Faezi1, Bahareh Mehramouz2, Sepehr Taghizadeh1, Pasquale Pagliano3, Hossein Samadi Kafil4
1Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran;
2Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran;
3Departement of Medicine, University of Salerno, Italy;
4Drug Applied Research Center, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Article received 1 July 2022, accepted 14 July 2022
Corresponding author
Hossein Samadi Kafil
E-mail: Kafilhs@tbzmed.ac.ir
SummaRY
Several clusters and individual cases of acute hepatitis have been reported in the US, Europe and recently in Asia and Central America since October 2021. A laboratory investigation of the common viral hepatitis agents (HAV, HBV, HCV, HDV and HEV) yielded negative results prompting the use of the term “acute non HepA-E hepatitis” to describe this condition. As of 24 June of 2022, WHO have reported 920 probable cases of severe acute hepatitis of unknown origin among pediatrics in 33 countries in five WHO regions. Since the previous reports on 27 May 2022, 270 new probable cases have been increased, including from four new countries, some of whom were also found to be positive for SARS-CoV-2. All the patients showed symptoms such as vomiting, diarrhea, jaundice, and abdominal pain. The patients’ liver enzymes were remarkably increased. No connection with SARS-CoV-2 or its vaccine has been found so far. However, the suspected cause is adenovirus, including its genomic variations, because its pathogenesis and laboratory investigations have been positively linked. Until further evidence emerges, hygiene precautions could be helpful to prevent its spread.
Keywords: hepatitis, non HepA-E hepatitis, viral hepatitis, liver, infection.
INTRODUCTION
Hepatitis is a condition characterized by inflammation of the liver parenchyma [1]. Inflammation may be acute, usually lasting less than six months, and subsequently normal liver function, or it may be chronic [2]. Non-infectious causes of hepatitis in children include immunological conditions (such as autoimmune diseases), metabolic diseases (such as Wilson’s disease, tyrosinemia), and exposure to toxins or drugs (such as acetaminophen). The most common infectious agents are primary liver viruses (hepatitis A, B, C, D, E). Other viruses that can cause acute hepatitis include: Epstein-Barr virus (EBV), cytomegalovirus (CMV), parvovirus, enteroviruses, adenoviruses, rubella virus, herpes viruses (HHV-1, HHV-2, HHV-6, HHV-7) and human immunodeficiency virus (HIV). Other infectious agents that may cause hepatitis include Brucella spp, Coxiella burnetii, and Leptospira [3].
Common symptoms of acute hepatitis include myalgia, nausea, vomiting, lethargy, fatigue, fever, abdominal pain, and diarrhea. These symptoms sometimes last for several weeks. A high proportion of acute infections with hepatitis viruses are asymptomatic, and for hepatitis A and B, children are much more likely to get an infection than adults, which can cause a minor or asymptomatic illness [4]. Jaundice is usually associated with acute hepatitis, but many cases of viral hepatitis may not show this feature. Death from acute viral hepatitis is rare and is usually the result of acute hepatitis, acute liver failure (ALF) with hepatic encephalopathy. The risk of ALF due to viral hepatitis is associated with aging and previous liver disease. Prolonged prothrombin coagulation disorder is one of the classic markers of ALF. Hepatic encephalopathy can be subtle, especially in infants. Bone marrow failure occurs in a small number of children with ALF, ranging from mild pancytopenia to aplastic anemia [5]. Without liver transplantation, mortality is very high in children with ALF. In more than 50% of cases of ALF in children, the cause is not identifiable and they are classified as indeterminate [6]. Treatment of indeterminate cases of ALF is general supportive measures and liver transplantation.
Acute hepatitis (Non Hepa A-E) of unknown origin refers to cases of severe hepatitis that is not caused by any of the five strains of the virus. Some children with acute and severe hepatitis of unknown origin become infected with a virus called adenovirus type 41 (which causes acute gastroenteritis). However, it is not clear whether the virus causes recent cases of hepatitis [7].
Human adenoviruses (HAdVs) are non-enveloped doubled-stranded DNA viruses that are common pathogens with worldwide distribution. They usually cause self-limiting infections in a healthy population. However, severe or diffuse HAdV infections may occur in some people, more commonly in immunocompromised patients [8-10]. More than 5 to 10 percent of all febrile illnesses in infants and young children are caused by HAdV, and almost all adults have serological evidence of previous infection with one or more HAdVs [11]. Infections present throughout the year without particular seasonality [12]. Epidemics occur globally, e.g. in closed or crowded settings or communities [13, 14]. Common transmissions include inhaling aerosol droplets, oral-fecal diffusion, or conjunctival insemination. Because the virus can survive on environmental surfaces for long periods of time, its absorption from external sources (such as pillows, sheets) has been described [15]. Additionally, HAdV reactivation may occur in immunocompromised patients [12]. Adenovirus type 41 usually causes acute gastroenteritis in children, which usually presents as diarrhea, vomiting, and fever. It is often accompanied with respiratory symptoms [16]. While there have been reports of hepatitis in children with immunodeficiency associated with adenovirus type 41 infection, adenovirus type 41 has not been identified as a cause of hepatitis in healthy children [17,18]. Hepatitis in association with Human adenoviruses (HAdV) infection has been reported in young infants, mainly in children with an overwhelming disseminated disease or in immunocompromised patients [19, 20]. Few cases of HAdV hepatitis have been reported in immunocompromised pediatric patients. Adenoviral hepatitis can occur sporadically secondary to liver transplantation or by the spread of the virus through the bloodstream to the liver. In transplants, it may be directly related to a transplanted liver infection or reactivation of the virus from a latent source. Focal inflammatory infiltrates are seen along with necrosis of normal liver cells and spotted cells. Pathologic diagnosis can be confirmed by PCR, IHC, and thin section transmission EM [21].
CURRENT GEOGRAPHIC DISTRIBUTION OF THE REPORTED ACUTE NON HEPA-E HEPATITIS CASES
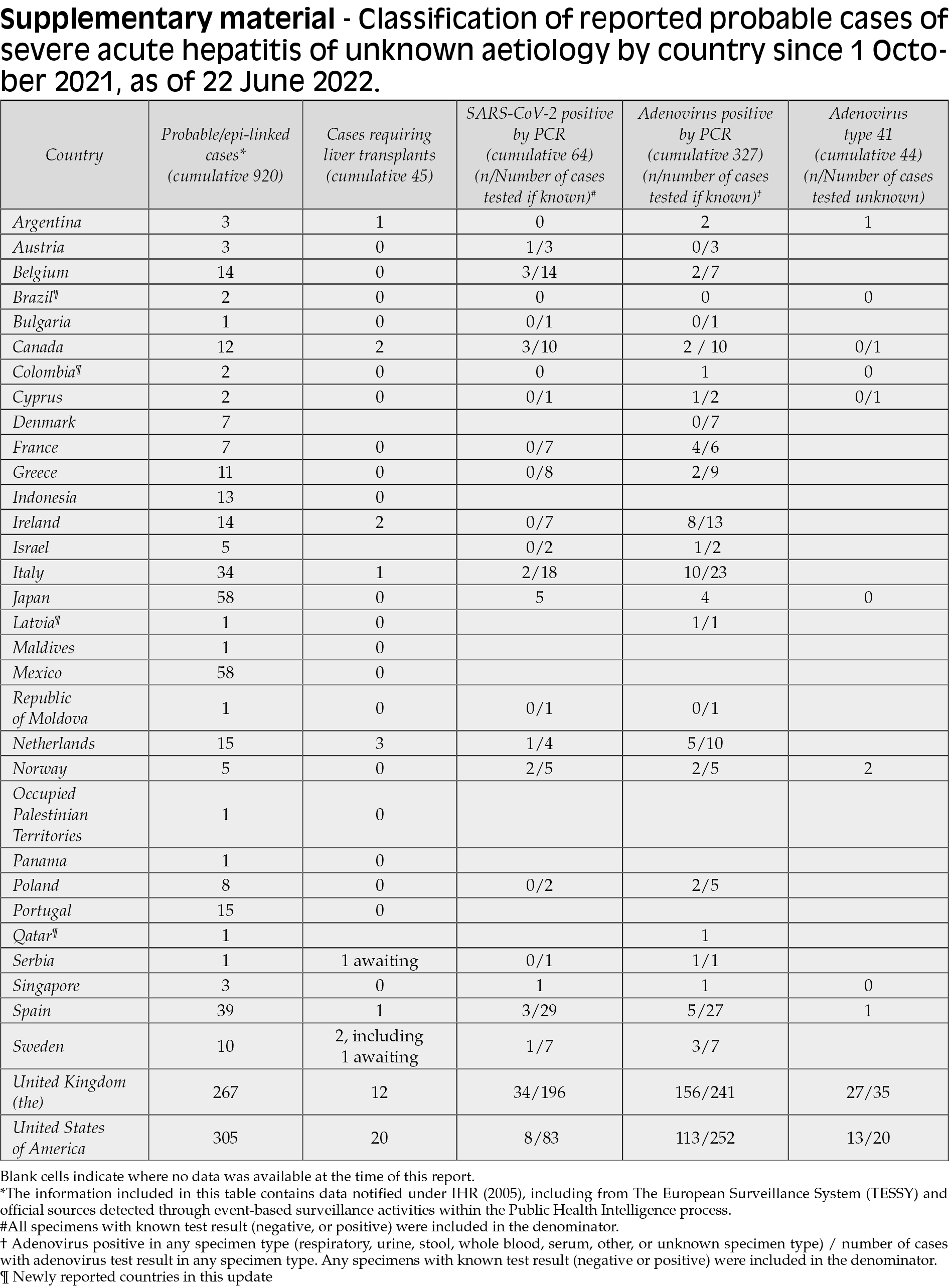
As of 22 June of 2022, WHO have reported 920 probable cases of severe acute hepatitis of unknown origin among pediatrics in 33 countries in five WHO regions. (Figure 1). These include new and retrospectively identified cases since 1 October 2021, which fit the WHO case definition as stated below. Since the previous reports on 27 May 2022, 270 new probable cases have been increased, including from four new countries. The diagnosis of severe acute hepatitis of unknown aetiology in children across five WHO Regions is abnormal, and the severe clinical consequences of some cases require careful examination. This outbreak was initially detected on 5 April 2022 when the United Kingdom of Great Britain and Northern Ireland (the United Kingdom) notified WHO of 10 cases of severe acute hepatitis of unknown aetiology in previously healthy young children aged under 10 years in the central belt of Scotland. Four other countries have reported cases awaiting classification that have not been included in the cumulative count. Of the probable cases, 45 children (5%) needed a transplant and 18 (2%) deaths were reported to the WHO [22]. Half of the probable cases reported from the WHO European Area (20 countries reported 460 cases), including 267 cases (29% of global cases) from the UK (Table 1, Figure 2). The second highest number of cases was reported from the Americas (n=383, including 305 from the United States), followed by the Pacific West (n=61) and Southeast Asia (n=14) and the Eastern Mediterranean region (n=2). Seventeen countries have reported more than five possible cases. The actual number of cases may be underestimated, in part because of the limited advanced surveillance schemes available. The number of cases is expected to change as more information and verified data become available [22].
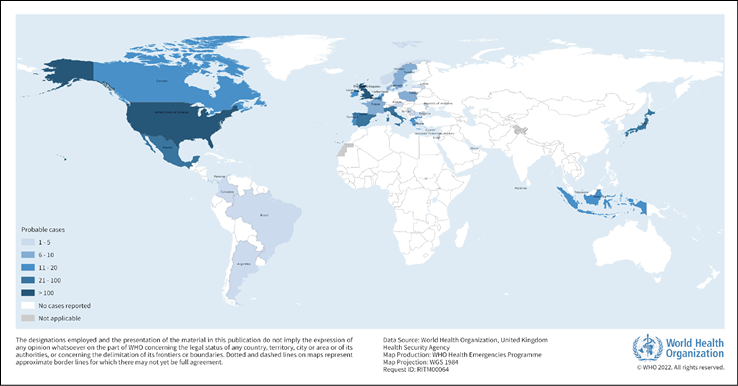
Figure 1 - Distribution of probable cases of severe acute hepatitis of unknown aetiology in children by country, as of 22 June 2022 (n=920).
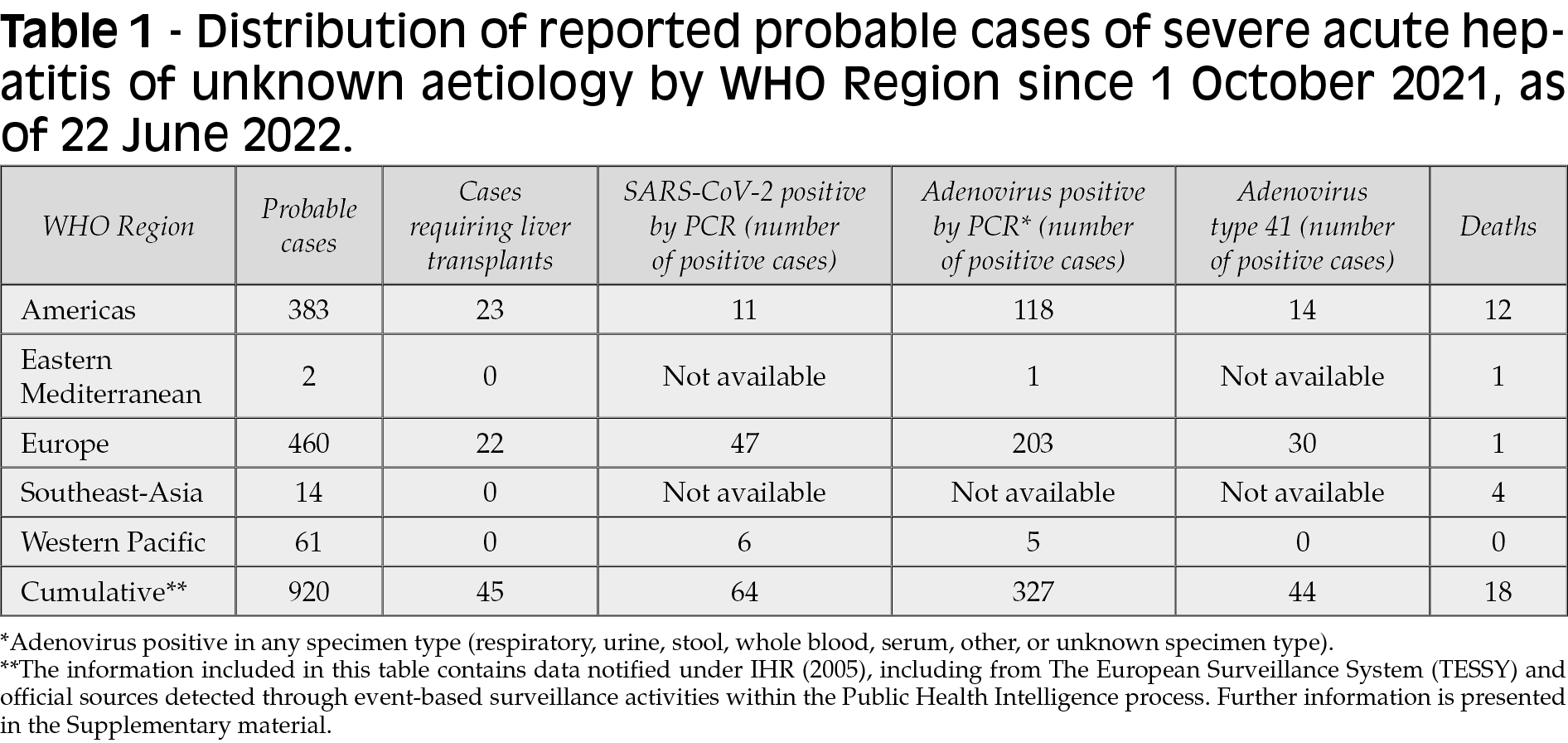
Figure 2 - Epidemiological curve of probable cases of severe acute hepatitis of unknown aetiology with available data, by week, by WHO region, as of 22 June 2022 (n=476). Note: The figure only includes cases for which dates of symptom onset, hospitalization, or notification were reported to WHO (n=476). The date of symptom onset was used when available (n=289). If unavailable, the week of hospitalization (n=163), or the week of notification (n=24), was used.
The United Kingdom Health Security Agency (UKHSA) continues to investigate and confirm cases of sudden onset of hepatitis in 10 years of age and younger children which identified since January 2022. Working alongside with Scotland Public Health, Wales Public Health and the Public Health Agency active investigations has identified seven more confirmed cases since the last update on 17 June, bringing the total number of confirmed cases in the UK to 258 by 21 June. Of those confirmed cases, 183 are residents of the UK, 35 in Scotland, 18 in Wales and 22 in Northern Ireland. These cases are mainly in children under 5 years’ old who showed the first signs of gastroenteritis (diarrhea and nausea) and then the onset of jaundice. In part of the research, a small number of children over the age of 10 are also being considered as possible cases. No children have died [23].
LABORATORY TESTING OF CASES
Laboratory testing for hepatitis A-E virus in these children excluded the possible definition (Box 1). Other pathogens have been identified in a number of cases, although data reported to the WHO are incomplete.

Box 1 - WHO Working case definition of acute hepatitis of unknown aetiology.
Adenovirus is still the most commonly identified pathogen among the available data. In the European region, adenovirus was detected by PCR in 55% of cases (203/371) with available results (see Supplementary material). Preliminary reports from the United States show that adenovirus has been detected in 45% of cases (253/113) with the available results.
SARS-CoV-2 has been identified in a number of cases, however, data on serological outcomes are limited. In the European region, SARS-CoV-2 was detected by PCR in 15% of cases (47/307) with available results (see Annex). Preliminary reports from the United States show that SARS-CoV-2 is detected in 10% of cases (8/83) with available results. Most cases reported do not appear to be epidemiologically linked. However, epidemiologically related cases have been reported in Scotland and the Netherlands [22].
EPIDEMIOLOGICAL CHARACTERISTICS OF CASES
As of June 22, 2022, out of 422 cases with sexual and age information, 48% of cases were male (n=202) and the majority of cases (78%, n=327) were under 6 years old (Figure 3). Of the possible 100 cases with available clinical data, the most commonly reported symptoms were nausea or vomiting (54% of cases), jaundice (49% of cases), general weakness (45% of cases), and abdominal pain (45% of cases).
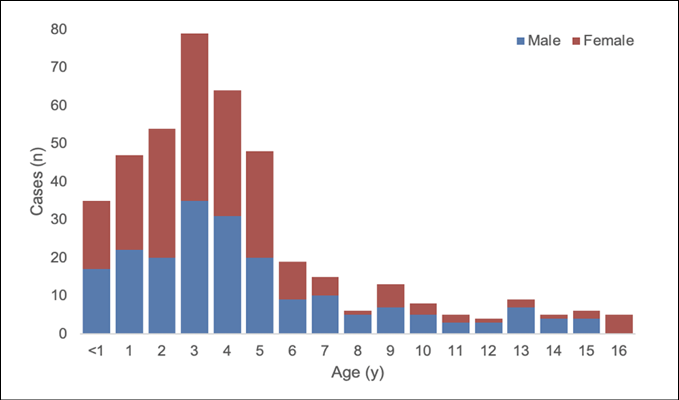
Figure 3 - Age and gender distribution of reported probable cases of severe acute hepatitis of unknown aetiology with available data (n=422).
Of the global cases, a total of 141 cases had the date of onset of symptoms and the date of hospitalization with available data. The mean number of days between the date of onset of symptoms and the date of hospitalization was four days [interquartile range (IQR) 7] [22].
LABORATORY TESTING
Depending on the clinical manifestations, suitable specimens for diagnosis include feces, respiratory specimens (such as nasopharyngeal swabs, nasopharyngeal/tracheal aspiration, alveolar bronchial lavage), conjunctival swabs, urine, genital secretions, and biopsy specimens (e.g. of liver or spleen). Isolation of the virus from the blood provides strong evidence of invasive or diffuse disease. Adenovirus infection can be diagnosed using a variety of tests, including antigen detection, polymerase chain reaction (PCR), virus isolation, and serology. PCR is the most common way to diagnose the virus in respiratory, fecal, blood, urine, or other specimens [7].
Typing can be done using specific type monoclonal antibodies (commercially available reagents) or using molecular methods (such as PCR and sequencing). Different genome types in serotypes are identified by restriction enzyme analysis, multiplex PCR or sequencing techniques that target the AdV fiber and hexon genes. Whole Genome Sequencing (WGS) has made it possible to spread information about the genetic structure of AdV. WGS has been used, for example, to detect recombination between different types of AdV [7]. Serology can detect a significant increase in antibody levels between serum samples collected during acute illness and recovery two to four weeks later. Serological methods are not used as first-line diagnostic methods. Intermittent and/or persistent excretion of adenovirus after acute infection is common, which makes the clinical interpretation of a positive molecular test challenging [24]. In addition, there are reports that adenovirus infection is difficult to confirm by histopathology [25, 26].
According to WHO recommendations, priority should be given to routinely collecting different samples from the earliest possible time after the onset of symptoms so that further tests can be performed if necessary and the cause(s) identified. If laboratory capacity is limited, storage and referral to regional or global laboratories for proposed research diagnostics should be considered. Any positive samples should also be stored for further testing and / or investigation [22].
POTENTIAL CONTROL MEASURES
Provided that human intestinal adenovirus infection remains the most likely cause of these acute hepatitis cases, close contact with an infected person should be considered the most likely route of exposure. Oral transmission through feces should be considered the most likely route of transmission, especially in young children and particularly as regards HAdV 41 [27]. However, as current evidence for aetiology and transmission is poor, the recommended measures should reflect good hygienic practice. Hand hygiene and respiratory etiquette should be observed in kindergartens experiencing gastroenteritis. Single use gloves should be considered for changing staff diapers, followed by hand hygiene. Complete disinfection of surfaces must be performed [3]. In the health care sector, standard and contact precautions should be followed for all possible and approved cases, and if there are respiratory symptoms, respiratory precautions should be added. In hospitals with potential cases of acute hepatitis, as defined above, patient transfer or relocation of staff between different hospital units should be restricted to prevent transmission. The possibility of co-occurring acute hepatitis with other patients should also be avoided. Adenoviruses can survive on surfaces and foams such as towels and are not easily inactivated by alcohol-based hand gels or even hand washing. Disinfection of medical equipment may require higher concentration bleach solutions (e.g. 10%) or other high-level disinfectant products [3].
Until more is known about the aetiology of these cases, WHO advises implementation of general infection prevention and control practices including: performing frequent hand hygiene, using soap and water or an alcohol-based hand-gel, avoiding crowded spaces and maintain a distance from others, ensuring good ventilation when indoors, wearing a well-fitted mask covering your mouth and nose when appropriate, covering coughs and sneezes, using safe water for drinking, following the Five Keys to Safer Food:
1) keep clean;
2) separate raw and cooked;
3) cook thoroughly;
4) keep food at safe temperatures; and
5) use safe water and raw materials, regular cleaning of frequently touched surfaces, and staying home when unwell and seeking medical attention.
Health centers should follow standard precautions and apply contact and drop precautions for suspected or possible cases [22].
HYPOTHESES OF POSSIBLE ETIOLOGY
Adenovirus role
Currently, the most plausible hypothesis to explain cases of acute non HepA-E hepatitis among children entails the role of adenovirus infection [3, 23, 28]. In the technical briefing by the UK Health Security Agency, a working hypothesis with best fits to regulatory data assumes that natural adenovirus infection in children can be complicated by a co-factor that severely transforms the infection or can cause immunopathology [23]. Possible co-factors include the issue of higher susceptibility as a result of less exposure to adenoviruses during the 2019 coronavirus pandemic (COVID-19) with widespread acceptance of non-pharmacological interventions and consequent reduction in exposure to various pathogens [29, 30]. Thus, restrictions imposed amid the ongoing COVID-19 pandemic may lead to later exposure of young children to adenoviruses, with delayed exposure leading to a more severe immune response that causes severe liver damage [23]. Such a scenario has been proposed by Ruben H de Kleine et al. who reported a preliminary absence of a notable increase in pediatric acute liver failure upon comparing the data for the years 2019-2021 with the data for the first 4 months of 2022 [31]. Other possible co-factors include previous or concomitant infection with other viruses, including acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or exposure to toxins or other environmental agents [23, 28]. Another hypothesis to explain acute non-HepA-E hepatitis includes the role of adenovirus as well. However, this hypothesis assumes that a new type of adenovirus is the main cause with or without the aforementioned co-factors [23, 28]. The argument in favor of the role of adenoviruses in acute non-HepA-E hepatitis is based on the observation that more than three-quarters of the reported cases for adenoviruses were positive [3, 23, 28]. Scientists and clinicians are advised to check if there is a change in the genome of the virus that may cause hepatotropism and severe inflammation of the liver. An important point to consider is the report of acute non-HepA-E hepatitis amid the COVID-19 pandemic. The disease has been associated with increased molecular testing capacity for viruses worldwide. Therefore, the diagnosis of previously unknown cases of HAdV-F41-associated hepatitis exacerbated by delayed exposure to this common infection may be an acceptable hypothesis that needs further confirmation [32, 33].
COVID-19
One of the hypotheses currently being investigated for the origin of acute non-hepA-E hepatitis is the possible role of SARS-CoV-2 infection [23]. In particular, a new strain of the virus could play a role. However, given the absence of SARS-CoV-2 positively (active or previous) in most cases, such a hypothesis does not seem plausible. Specifically, eight of the 13 cases reported in Scotland were negative for SARS-CoV-2 by polymerase chain reaction (PCR), and the other two were positive 3 months or earlier prior to admission [34]. In addition, all 9 Alabama tests were negative for SARS-CoV-2, minimizing the possibility of a direct role for COVID-19 in acute non-A-E hepatitis [35]. Moreover, acute hepatitis has not been a common feature of COVID-19 in children, despite reports of its possible occurrence [36, 37]. Variants that escape the currently available testing modalities for molecular detection appear unlikely as well, considering the previous evidence showing that the diagnostic accuracy of the PCR was not impacted by emerging SARS-CoV-2 variants, including the most recent dominant genetic lineage, namely, omicron [38, 39].
COVID-19 Vaccination
Although the possibility of an association between acute cases of non-A-E hepatitis and side effects after COVID-19 vaccination has been considered, such a hypothesis seems far-fetched given that the majority of cases occurred in children that were not vaccinated against COVID-19 [28,40]. As the current reports, although rare, pointed to the prevalence of acute non-A-E hepatitis in young children - with children 5 years of age or younger who are not eligible for COVID-19 vaccination - these observations almost rule out the role of Covid-19 vaccination in this emerging issue [41].
CONCLUSIONS
Currently, since the underlying cause of this disease is still unknown, it is recommended that suspected patients be quarantined during diagnostic and therapeutic procedures. Objects contaminated with body fluids, feces, excrements or blood must be thoroughly disinfected [33]. After the initial evaluation of the patient, the case should be reported to the adjacent health department. After obtaining relevant patient information, based on signs and symptoms and laboratory reports, a multidisciplinary team consisting of pediatricians, infectious diseases, emergency medical and intensive care physicians should be immediately beginning further diagnosis and treatment for severe hepatitis of unknown. It is currently difficult to verify whether similar cases of hepatitis have occurred in Europe due to the lack of comprehensive monitoring and study of hepatitis caused by human adenovirus infection. In addition, it is currently difficult to perform human adenovirus viral monitoring based on clinical signs, and the potential risk of human adenovirus-associated hepatitis should be assessed as soon as possible using relevant epidemiological, clinical, and virological data. Also information on risk factors, to provide scientific and technical support for the prevention and control of this disease [32, 42]. Epidemiological, clinical, laboratory, histopathological and toxicological investigations of the possible aetiology (or aetiologies) of the cases are underway by several national authorities, research networks, across different working groups in WHO and with partners. This includes detailed epidemiological investigations to identify common exposures, risk factors or links between cases. Additional investigations are also planned to ascertain where the number of detected cases are above expected baseline levels [22].
Acknowledgments
This study was supported by Tabriz University of Medical Sciences (Drug Applied Research center). This is a literature review report and did not need to get ethical approve. All data are available based on communication with corresponding author.
Conflict of interest
None to declare
Funding
None
REFERENCES
[1] Schaefer TJ, John S. Acute hepatitis. In: StatPearls [Internet]: StatPearls Publishing. 2021.
[2] Chugh A, Maximos M, Perlman M, et al. Viral Hepatitis in Children: A Through E. Pediatr annal. 2016; 45 (12), e420-e426.
[3] European Centre for Disease Prevention and Control. Increase in severe acute hepatitis cases of unknown aetiology in children. 2022.
[4] Ryder S, Beckingham I. Acute hepatitis. Brit Med J. 2001; 322 (7279), 151-153.
[5] Patel KR, Bertuch A, Sasa GS, et al. Features of hepatitis in hepatitis-associated aplastic anemia: clinical and histopathologic study. J Pediatr Gastroenterol Nutr. 2017; 64 (1), e7-e12.
[6] Lu BR, Zhang S, Narkewicz MR, et al. Evaluation of the liver injury unit scoring system to predict survival in a multinational study of pediatric acute liver failure. J Pediatr. 2013; 162 (5), 1010-1016. e1014.
[7] Lynch III JP, Kajon AE. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. In: Seminars in respiratory and critical care medicine: Thieme Medical Publishers. 2016; 586-602.
[8] Gu J, Su Q-q, Zuo T-t, et al. Adenovirus diseases: a systematic review and meta-analysis of 228 case reports. Infection. 2021; 49 (1), 1-13.
[9] Kim Y-J, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. In: Sem RespCrit Care Med. Copyright© 2007 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New York. 2007; 222-242.
[10] Fox JP, Hall CE, Cooney MK. The Seattle virus watch: VII. Observations of adenovirus infections. Am J Epidemiol. 1977; 105 (4), 362-386.
[11] Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis. 2006; 43 (3), 331-339.
[12] Kolavic-Gray SA, Binn LN, Sanchez JL, et al. Large epidemic of adenovirus type 4 infection among military trainees: epidemiological, clinical, and laboratory studies. Clin Infect Dis. 2002; 35 (7), 808-818.
[13] Kujawski SA, Lu X, Schneider E, et al. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018–2019. Clin Infect Dis. 2021; 72 (11): 1992-1999.
[14] Russell KL, Broderick MP, Franklin SE, et al. Transmission dynamics and prospective environmental sampling of adenovirus in a military recruit setting. J Infect Dis. 2006; 194 (7), 877-885.
[15] Kang G. Viral diarrhea. International Encyclopedia of Public Health. 2017; 360-367. doi: 10.1016/B978-0-12-803678-5.00486-0.
[16] Munoz RE, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. 1998; 27 (5): 1194-1200.
[17] Peled N, Nakar C, Huberman H, et al. Adenovirus infection in hospitalized immunocompetent children. Clin Pediatr. 2004; 43 (3), 223-229.
[18] Onda Y, Kanda J, Sakamoto S, et al. Detection of adenovirus hepatitis and acute liver failure in allogeneic hematopoietic stem cell transplant patients. Transpl Infec Dis. 2021; 23 (2): e13496.
[19] Kim YJ, Schmidt NJ, Mirkovic RR. Isolation of an intermediate type of adenovirus from a child with fulminant hepatitis. J Infect Dis. 1985; 152 (4): 844. doi: 10.1093/infdis/152.4.844
[20] Shieh W-J. Human adenovirus infections in pediatric population-an update on clinico-pathologic correlation. Biomed J. 2021; 45 (1), 38-49. doi: 10.1016/j.bj.2021.08.009.
[21] WHO. World Health Organization (WHO). Multi-Country-Acute, Severe Hepatitis of Unknown Origin in Children Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON394; 2022 24 June.
[22] Agency UHS. Increase in hepatitis (liver inflammation) cases in children under investigation. London: UKHSA; 2022. Available at: https://www.gov.uk/government/news/hepatitis-liver-inflammation-cases-in-children-latest-updates 23 June 2022.
[23] Kimberlin DW. Red Book: 2018-2021 report of the committee on infectious diseases. Am acad pediatr 2018.
[24] Matoq A, Salahuddin A. Acute hepatitis and pancytopenia in healthy infant with adenovirus. Case Rep Pediatr. 2016; 2016, 8648190. doi: 10.1155/2016/8648190.
[25] Canan O, Ozçay F, Bilezikçi B. Adenovirus infection as possible cause of acute liver failure in a healthy child: a case report. Turkish J Gastroenter. 2008; 19 (4), 281-283.
[26] Quah S. International encyclopedia of public health. Academic Press. 2016.
[27] WHO. Multi-Country-Acute, severe hepatitis of unknown origin in children. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376 (accessed on 28 April 2022). In; 2022 28 April.
[28] Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021; 12 (1), 1-7.
[29] Khodadadi E, Maroufi P, Khodadadi E, et al. Study of combining virtual screening and antiviral treatments of the Sars-CoV-2 (Covid-19). Microb Pathogen. 2020; 145 (2020).
[30] de Kleine RH, Lexmond WS, Buescher G, et al. Severe acute hepatitis and acute liver failure of unknown origin in children: a questionnaire-based study within 34 paediatric liver centres in 22 European countries and Israel, April 2022. Eur Surveill. 2022; 27 (19), 2200369.
[31] Sallam M, Mahafzah A, Şahin GÖ. Hepatitis of Unknown Origin and Etiology (Acute Non HepA-E Hepatitis) among Children in 2021/2022: Review of the Current Findings. In: Healthcare: Multidisciplinary Digital Publishing Institute; 2022; 973.
[32] Mattner F, Sykora K-W, Meissner B, et al. An adenovirus type F41 outbreak in a pediatric bone marrow transplant unit: analysis of clinical impact and preventive strategies. Pediatr Infect Dis J. 2008; 27 (5), 419-424.
[33] Marsh K, Tayler R, Pollock L, et al. Investigation into cases of hepatitis of unknown aetiology among young children, Scotland, 1 January 2022 to 12 April 2022. Euro Surveill. 2022; 27 (15), 2200318.
[34] Baker JM, Buchfellner M, Britt W, et al. Acute hepatitis and adenovirus infection among children-Alabama, October 2021-February 2022. MMWR. 2022; 71 (18), 638.
[35] Brisca G, Mallamaci M, Tardini G, et al. SARS-CoV-2 infection may present as acute hepatitis in children. Pediatr Infect Dis J. 2021; 40 (5), e214-e215.
[36] Ozma MA, Maroufi P, Khodadadi E, et al. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (Covid-19) during the outbreak period. Infez Med. 2020; 28 (2), 153-165.
[37] WHO. Enhancing Response to Omicron SARS-CoV-2 Variant. Available online: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical brief-and-priority-actions-for-member-states (accessed on 29 April 2022). In; 2022 29 April.
[38] Fathizadeh H, Taghizadeh S, Safari R, et al. Study presence of COVID-19 (SARS-CoV-2) in the sweat of patients infected with Covid-19. Microb Pathogen. 2020; 149, 104556-104556.
[39] Mücke MM, Zeuzem S. The recent outbreak of acute severe hepatitis in children of unknown origin-what is known so far. J Hepatol. 2022; 77 (1), 237-242. doi: 10.1016/j.jhep.2022.05.001.
[40] WHO. Coronavirus Disease (COVID-19): Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a detail/coronavirus-disease-(covid-19)-vaccines (accessed on 29 April 2022). 2022 29 April.
[41] Rabaan AA, Bakhrebah MA, Nassar MS, et al. Suspected adenovirus causing an emerging hepatitis among children below 10 years: a review. Pathogen. 2022; 11 (7), 712.