Le Infezioni in Medicina, n. 3, 459-463, 2022
doi: 10.53854/liim-3003-16
CASE REPORT
Actinomycosis mimicking malignancy: a report of three cases diagnosed with fine-needle aspiration cytology
Pasquale Cretella, Maria Carola Italia, Bianca Serio, Pio Zeppa, Alessandro Caputo
Department of Medicine and Surgery, University of Salerno, Salerno, Italy
Article received 4 June 2022, accepted 16 July 2022
Corresponding author
Pio Zeppa
E-mail: pzeppa@unisa.it
SummaRY
We describe three cases of actinomycosis of the head and neck area, clinically suspected to be malignancies, diagnosed by fine-needle aspiration (FNAC). The patients presented with painless, slowly growing masses in the cervicofacial area. Ultrasonography identified the masses as enlarged lymph nodes which were subsequently biopsied by FNAC. Cytological features were similar in all cases, with a background of granulocytes and scattered lymphocytes and histiocytes. At high magnification colonies of branching, filamentous and beaded bacteria were detected. In the Diff-Quik–stained smears, these filamentous colonies showed an evident yellowish color with the typical feature of the “sulfur granules” consistent with the Splendore-Hoeppli phenomenon. A diagnosis of actinomycosis was made and confirmed in all cases by the subsequent microbiological tests. The patients were treated with high-dose penicillin, which caused the masses to progressively shrink. The lymph nodal localization of cervico-facial actinomycosis may be a diagnostic challenge, because in that area, lymphadenopathies may occur both in benign and malignant conditions. FNAC is a safe, fast, and reliable method to perform an accurate diagnosis of actinomycosis avoiding the surgical excision for histological evaluation.
Keywords: actinomycosis, fine-needle aspiration, lymphadenopathy.
Actinomycosis is a rare chronic disease caused by Actinomyces spp., anaerobic Gram-positive bacteria that normally colonize the human digestive and genital tracts. The most common member of the family is Actinomyces israelii [1]. Typical clinical presentations are cervicofacial actinomycosis following dental focus of infection, pelvic actinomycosis in women with an intrauterine device, and pulmonary actinomycosis in individuals with poor dental hygiene. A break in the integrity of mucosal tissues is required to cause the disease, and usually a streptococcal or staphylococcal infection coexists [1, 2]. Young adults, especially males, represent the most affected population. The histologic picture includes a granulomatous, fibrous reaction with formation of abscesses which may contain the so-called “sulfur granules”, composed of Actinomyces, other bacterial species, and proteinaceous material [1, 3]. Furthermore, the presence of Actinomyces is associated with the Splendore-Hoeppli phenomenon, which consists in the presence of eosinophilic structures composed of necrotic debris and immunoglobulins which form rings around the bacterial granules [3]. Cervicofacial area involvement is characterized by palpable lesions (“humps and bumps”). Lymph node involvement may lead to misdiagnosis of other granulomatous conditions (i.e., tuberculosis) and even of neoplastic processes [2, 4, 5]. Culture is believed to be the definitive means of identification, but it is successful in less than half of the cases. Although histopathological examination is the most used diagnostic method, fine needle aspiration cytology (FNAC) has emerged as a simpler alternative. FNAC has a role in the diagnosis of both reactive and neoplastic lymphadenopathies, and FNAC features of actinomycosis have been described [3, 4, 6-11]. In this article we report three cases of actinomycosis with cervicofacial lymphadenopathy which were clinically suspicious for neoplasia.
We present the cases of three women referred to our Cytopathology Unit for cervical lymphadenopathy. The first woman, aged 38, had a history of papillary thyroid carcinoma resected two years prior. The second woman, aged 48, had a history of dental and periodontal problems. The third woman, aged 52, had a history of oral squamous cell carcinoma resected one year before. All three women presented with a single lymphadenopathy that did not regress after clinical follow-up and antibiotic treatment and were thus prescribed a lymph nodal FNAC to exclude malignancy. All three lymph nodes had slowly increased in size, and at clinical examination they were painless and enlarged (Table 1). Ultrasonography (US) confirmed the lymph nodal nature of the masses, which appeared oval, with a preserved hilum and a thin cortex. The pattern was hypoechoic and heterogeneous. FNAC was performed and necrotic-suppurative material was collected. Rapid on-site evaluation was immediately performed. The cytological features were similar in all cases and are described together. Smears showed a background of granulocytes with scattered lymphocytes and histiocytes; lymphoid cells were present in all cases (Figure 1). At low magnification, colonies of branching, filamentous and beaded bacteria with neutrophils and eosinophils were observed (Figure 2). In the Diff-Quik–stained smears, these filamentous colonies showed an evident yellowish color with the typical feature of the “sulfur granules” consistent with the Splendore-Hoeppli phenomenon (Figure 3). According to the clinical and US features, a provisional cytological diagnosis of lymph nodal actinomycosis was made in all cases. Residual material was used to prepare a cellblock in one case, and in all three cases all residual material was referred to the microbiology laboratory for confirmation based on Actinomyces identification in culture. All three patients were treated with high-dose intravenous penicillin. After 2-3 weeks, the lymph nodes returned to almost normal size. The microbiological tests confirmed the cytological diagnoses in all cases.
Patients provided written informed consent and all potentially identifying data has been anonymized as far as possible.

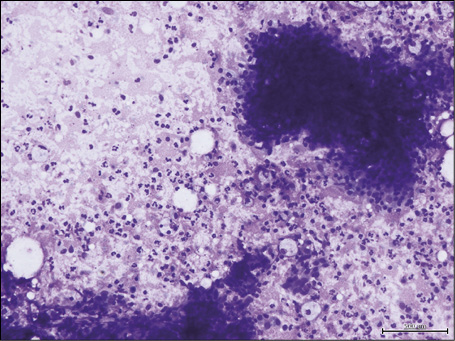
Figure 1 - Fine-needle aspiration cytology of lymph nodal actinomycosis: the smear shows a background of scattered granulocytes and lymphocytes; lymphoid aggregates assessing the lymph nodal nature of the aspirated nodule are present (Diff-Quik stain, 270x).
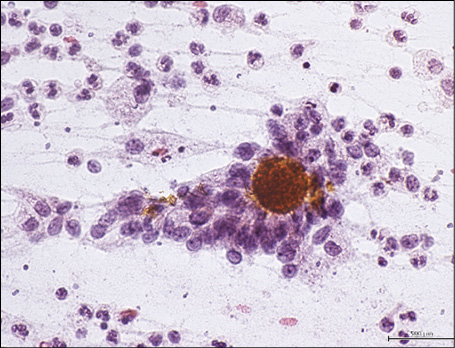
Figure 2 - Fine-needle aspiration cytology of lymph nodal actinomycosis showing colonies of yellowish branching, filamentous, beaded bacteria encircled by inflammatory cells (Papanicolaou stain, 430x).
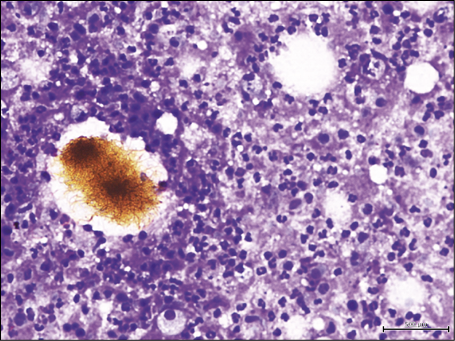
Figure 3 - Fine-needle aspiration cytology of lymph nodal actinomycosis showing filamentous colonies of bacteria with a yellowish color and the typical feature of “sulfur granules” consistent with the Splendore-Hoeppli phenomenon (Diff-Quik stain, 270X).
Cervico-facial swellings are the most common manifestation of actinomycosis accounting for 50-70% of reported cases [2]. Actinomycosis can occur at any age, with a male: female ratio of 3:1 and it is often the result of trauma, dental injuries, or caries [4,12,13]. The major complications of untreated actinomycosis are fistulae and invasion of the bloodstream, skull, or trachea [12]. Clinically, actinomycosis commonly presents as a variably tender nodular lesion usually localized at the angle of the jaw [2]. Lymphadenopathy is uncommon [1, 13, 14]. Imaging techniques such as US, computed tomography, and nuclear magnetic resonance provide only qualitative information (borders of lesion, homogeneity, localization, invasion of surrounding organs) [1, 5, 13, 14]. Definitive diagnosis can be obtained by demonstrating the presence of Actinomyces in culture; however, bacterial growth is slow and the definitive evaluation of the presence of Actinomyces may require 2 to 3 weeks [1, 2, 5, 13, 14]. FNAC is a safe, rapid, and relatively inexpensive method: it allows obtaining an amount of material sufficient to make a diagnosis with lower costs and distress for the patients, and is routinely used to solve diagnostic conundrums in the head and neck district [6, 7, 15-18]. FNAC can also be used to obtain material for bacterial cultures, histochemistry, immunohistochemistry, and molecular tests [12, 19-25]. Ancillary histochemical stains may be helpful to distinguish Actinomyces from its bacterial or non-bacterial mimics: by using Gomori silver and Gram stains, Actinomyces (positive) can be distinguished from its fungal mimickers, while a positive acid-fast stain will point towards a Nocardia infection [12]. A high-dose intensive antibiotic therapy is required for treating Actinomyces, due to its fibrous reaction resulting in hypovascularized tissue. The therapy consists in the administration of high-dose penicillin (Penicillin G - 12-24 million U/d IV by continuous infusion or in divided doses for 1-2 weeks, then switch to PO (penicillin VK) for 6-12 months) over a long period (6 months to 1 year), but a short course (< 6 months) of high-dose penicillin therapy has been proven to be effective in cervico-facial actinomycosis [1, 2, 12, 26]. Corticosteroids are used to reduce the residual inflammatory and granulomatous reaction [14]. Surgical treatment is required for resection of necrotic tissue and drainage of abscesses but, to prevent recurrence of disease, it must be followed by antibiotic treatment [5, 13].
Unfortunately, there is a little familiarity with this pathology because of its low incidence and the few reports in the international literature. Furthermore, often diagnosis is reached after extensive surgery and histological examination.
In conclusion, the lymph nodal localization of cervico-facial actinomycosis may be a diagnostic challenge, because in that area, lymphadenopathies may occur in both benign (e.g. granulomatous and suppurative infections) and malignant conditions (lymphoma, metastases). FNAC is a safe, fast, and reliable first-level test to exclude differential diagnoses and harvest material to perform an accurate diagnosis of actinomycosis.
Funding
This work received no specific funding.
Informed consent
The patients provided written informed consent for the procedures and for the publication of the present work. All potentially identifying data have been anonymized as far as possible.
Protection of human subjects and animals in research
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The Authors declare that they have no conflicts of interest to disclose.
REFERENCES
[1] Pulverer G., Schütt-Gerowitt H., Schaal K.P. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis. 2003; 37 (4), 490-497. doi: 10.1086/376621.
[2] Moniruddin A.B., Begum H., Nahar K. Actinomycosis: an update. Medicine today. 2010; 22 (1), 43-47.
[3] McHugh K.E., Sturgis C.D., Procop G.W., Rhoads D.D. The cytopathology of Actinomyces, Nocardia, and their mimickers. Diagn Cytopathol. 2017; 45 (12), 1105-1115. doi: 10.1002/dc.23816.
[4] Amrikachi M., Krishnan B., Finch C.J., Shahab I. Actinomyces and actinobacillus actinomycetemcomitans-Actinomyces-associated lymphadenopathy mimicking lymphoma. Arch Pathol Lab Med. 2000; 124 (10), 1502-1505. doi: 10.5858/2000-124-1502-AAAAAA.
[5] Lancella A., Abbate G., Foscolo A.M., Dosdegani R. Two unusual presentations of cervicofacial actinomycosis and review of the literature. Acta Otorhinolaryngol Ital. 2008; 28 (2), 89-93.
[6] Zeppa P. Haematocytopathology: why? Cytopathology. 2012; 23 (2), 73-75. doi: 10.1111/j.1365-2303.2012.00972.x.
[7] Caputo A., Ciliberti V., D’Antonio A, et al. Real-world experience with the Sydney System on 1458 cases of lymph node fine needle aspiration cytology. Cytopathology. 2022; 33 (2), 166-175. doi: 10.1111/cyt.13079.
[8] Hong I.S., Mezghebe H.M., Gaiter T.E., Lofton J. Actinomycosis of the neck: diagnosis by fine-needle aspiration biopsy. J Natl Med Assoc. 1993; 85 (2), 145-146.
[9] Thambi R., Devi L., Sheeja S., Poothiode U. Primary breast Actinomyces simulating malignancy: A case diagnosed by fine-needle aspiration cytology. J Cytol. 2012; 29 (3), 197-199. doi: 10.4103/0970-9371.101173.
[10] Gosavi A.V., Anvikar A.R., Sulhyan K.R., Manek D.D. Primary actinomycosis of breast-A diagnosis on cytology. Diagn Cytopathol. 2016; 44 (8), 693-695. doi: 10.1002/dc.23499.
[11] Aragao A., Biemer J., Barkan G.A., Pambuccian S.E. Splendore-Hoeppli phenomenon in a fine needle aspirate of cervicofacial actinomycosis. Diagn Cytopathol. 2019; 47 (3), 238-243. doi: 10.1002/dc.24025.
[12] Custal-Teixidor M., Trull-Gimbernat J.M., Garijo-López G., Valldosera-Rosello M. Fine-needle aspiration cytology in the diagnosis of cervicofacial actinomycosis: report of 15 cases. Med Oral Patol Oral Cir Bucal. 2004; 9 (5), 467.
[13] Volante M., Contucci A.M., Fantoni M., Ricci R., Galli J. Cervicofacial actinomycosis: still a difficult differential diagnosis. Acta Otorhinolaryngol Ital. 2005; 25 (2), 116-119.
[14] Bubbico L., Caratozzolo M., Nardi F., Ruoppolo G., Greco A., Venditti M. Actinomycosis of submandibular gland: an unusual presentation. Acta Otorhinolaryngol Ital. 2004; 24 (1), 37-39.
[15] Caputo A., Di Crescenzo R.M., Landolfi L., Zeppa P.. Lipoma of the tongue diagnosed by fine-needle aspiration cytology. Diagn Cytopathol. 2020; 48 (11), 1131-1133. doi: 10.1002/dc.24459.
[16] Zeppa P., Varone V., Cozzolino I., Salvatore D., Vetrani A., Palombini L. Fine needle cytology and flow cytometry of ectopic cervical thymoma: a case report. Acta Cytol. 2010; 54 (Suppl. 5), 998-1002.
[17] D’Antonio A, Baldi C, Memoli D, Caleo A, Rosamilio R, Zeppa P. Fine needle aspiration biopsy of intraparotid spindle cell lipoma: a case report. Diagn Cytopathol. 2013; 41 (2), 171-173. doi: 10.1002/dc.21801.
[19] Ronchi A, Di Martino M, Caputo A, et al. Fine-needle aspiration cytology is an effective diagnostic tool in paediatric patients with mucoepidermoid carcinoma as secondary neoplasm. Acta Cytol. 2020; 64 (6), 520-531. doi: 10.1159/000508395.
[19] Ronchi A, Caputo A, Pagliuca F, et al. Lymph node fine needle aspiration cytology (FNAC) in paediatric patients: Why not? Diagnostic accuracy of FNAC in a series of heterogeneous paediatric lymphadenopathies. Pathol Res Pract. 2021; 217, 153294. doi: 10.1016/j.prp.2020.153294.
[20] Petruzziello F., Zeppa P., Ciancia G., et al. Cytological and histological detection of amyloid deposits in bone marrow of patients affected by multiple myeloma. Leuk Lymphoma. 2011; 52 (12), 2304-2307. doi: 10.3109/10428194.2011.594192.
[21] Cozzolino I., Vitagliano G., Caputo A., et al. CD15, CD30, and PAX5 evaluation in Hodgkin’s lymphoma on fine-needle aspiration cytology samples. Diagn Cytopathol. 2020; 48 (3), 211-216. doi: 10.1002/dc.24366.
22. Vitagliano G., Cretella P., Zeppa P., Caputo A. Large-cell lymphoma with features intermediate between Hodgkin’s, primary mediastinal B-cell, and grey-zone lymphoma: a conundrum on fine needle aspiration cytology. Cytopathology. 2020; 31 (4), 325-328. doi: 10.1111/cyt.12865.
[23] Caputo A, D’Ardia A, Sabbatino F, et al. Testing EGFR with Idylla on Cytological Specimens of Lung Cancer: A Review. Int J Mol Sci. 2021; 22 (9), 4852. Published 2021 May 3. doi: 10.3390/ijms22094852.
[24] D’Ardia A., Caputo A., Fumo R., et al. Advanced non-small cell lung cancer: Rapid evaluation of EGFR status on fine-needle cytology samples using Idylla. Pathol Res Pract. 2021; 224, 153547. doi: 10.1016/j.prp.2021.153547.
[25] Cozzolino I., Giudice V., Mignogna C., Selleri C., Caputo A., Zeppa P. Lymph node fine-needle cytology in the era of personalised medicine. Is there a role? Cytopathology. 2019;30(4):348-362. doi: 10.1111/cyt.12708.
[26] Sudhakar, Selvin S., Ross, John J. Short-term treatment of actinomycosis: two cases and a review. Clinical infectious diseases. 2004; 38.3, 444-447.