Le Infezioni in Medicina, n. 3, 454-458, 2022
doi: 10.53854/liim-3003-15
CASE REPORT
Treatment with cefiderocol in K. pneumoniae KPC nosocomial external ventricular drainage meningitis: A brief report
Francesco Colombo1, Ali Waheed1, Sandro Panese2, Claudio Scarparo3, Maria Solinas3, Saverio Giuseppe Parisi1, Nicholas Geremia2
1Department of Molecular Medicine, University of Padova, Padua, Italy;
2Unit of Infectious Diseases, Department of Clinical Medicine, Ospedale dell’Angelo, Venice, Italy;
3Unit of Microbiology and Virology, Ospedale dell’Angelo, Department of Medical Direction, Ospedale “dell’Angelo”, Venice, Italy
Article received 9 June 2022, accepted 17 July 2022
Corresponding author
Francesco Colombo
E-mail: francesco.colombo@studenti.unipd.it
SummaRY
We report the case of successful use of cefiderocol (FDC) in a Carbapenemase Producing K. pneumoniae (CPKP) post-surgical meningitis in a 44-year-old man treated with antimicrobial therapy and external ventricular drainage (EVD). The patient was known for being colonised by CPKP; for this reason, therapy with ceftazidime/avibactam (CZA) plus fosfomycin and linezolid was started. After an initial response a CZA resistant CPKP strain was isolated from CSF culture, so the antibiotic therapy was modified to FDC with trimethoprim/sulfamethoxazole for 14 days, and EVD was replachttpd. A complete recovery was obtained. This is the first case report describing FDC administration in CPKP meningitis.
Keywords: cefiderocol, meningitidis, enterobacterales, Klebsiella pneumoniae, carbapenemase.
INTRODUCTION
Klebsiella pneumoniae is a human commensal and opportunistic pathogen that has become an important causative agent of hospital-acquired infections over the past few years. The emergence and global expansion of multidrug-resistant (MDR) clones of K. pneumoniae have been increasingly reported and represents an escalating public health threat [1, 2].
Ceftadizidme/avibactam (CZA) is a combination of the third-generation cephalosporin ceftazidime and a novel, non-β-lactam β-lactamase inhibitor avibactam with a good activity against Carbapenemase Producing Klebsiella pneumoniae [3, 4].
However, selective pressure of antimicrobial administration could lead to resistance even to novel molecules like CZA.
Cefiderocol (FDC) is a new broad-spectrum siderophore cephalosporin against Gram-negative bacteria, including carbapenem-resistant pathogens, which could represent a valid alternative to those strains that no longer show susceptibility to CZA [5].
CASE REPORT
A 44-year-old man was admitted to the neurosurgery ward of Ospedale dell’Angelo (Venice, Italy) with severe polytrauma following a road traffic accident. He required a cervical laminectomy and facial fracture stabilisation. He was found to be colonised with Carbapenemase Producing K. pneumoniae (CPKP) from his colorectal screening swabs after eleven days of hospitalisation. After treatment of his polytrauma, he was later discharged to a rehabilitation clinic for continuation of medical care. Two weeks after the discharge, a deterioration of his neurological status occurred with increasing drowsiness, confusion, disorientation, aphasia, and third cranial nerve palsy. He was thus re-admitted to neurosurgery ward.
Routine investigations during this re-admission showed moderate white blood cell count increase and rising C-reactive protein levels. In addition, screening swabs confirmed rectal colonisation with CPKP.
Along with this progressive neurological worsening, a cranial CT scan showed periventricular hypodensity consistent with transependymal transudation. At the base of the frontal hemisphere, the parenchyma appeared thinned and showed radiological features consistent with frontal extradural pneumocephalus. Tetraventricular hydrocephalus was also present.
Furthermore, the patient underwent a brain magnetic resonance imaging (MRI) investigation, which confirmed tetraventricular hydrocephalus with hyperintensity of signal around lateral ventricles, consistent with transependymal transudation, and a possible ethmoid sinus fistula associated with pneumocephalus was identified.
Two weeks later, the patient developed fever and worsening of neurological status, therefore lumbar puncture was performed. Yellowish-turbid cerebrospinal fluid (CSF) was obtained. Analysis of CSF showed low glucose level (6 mg/dL), high protein (731 mg/dL), lactate (8.20 mmol/L), and significantly raised white cell counts (1230 cell/mmc - 56% polymorphonuclear neutrophils vs 44% mononuclear leukocytes). CSF microscopy, culture, and bacterial nucleic acid amplification tests were performed. In addition, a blood culture was also carried out during a feverish peak.
Empirical therapy was started with CZA at the dosage of 2.5 g every 8 hours with an extended infusion of 3 hours plus, phosphomycin (FOS) at the dosage of 6 g every 6 hours plus, linezolid at the dosage of 600 mg every 12 hours. In addition, an external ventricular drainage (EVD) was placed as an emergency treatment of hydrocephalus.
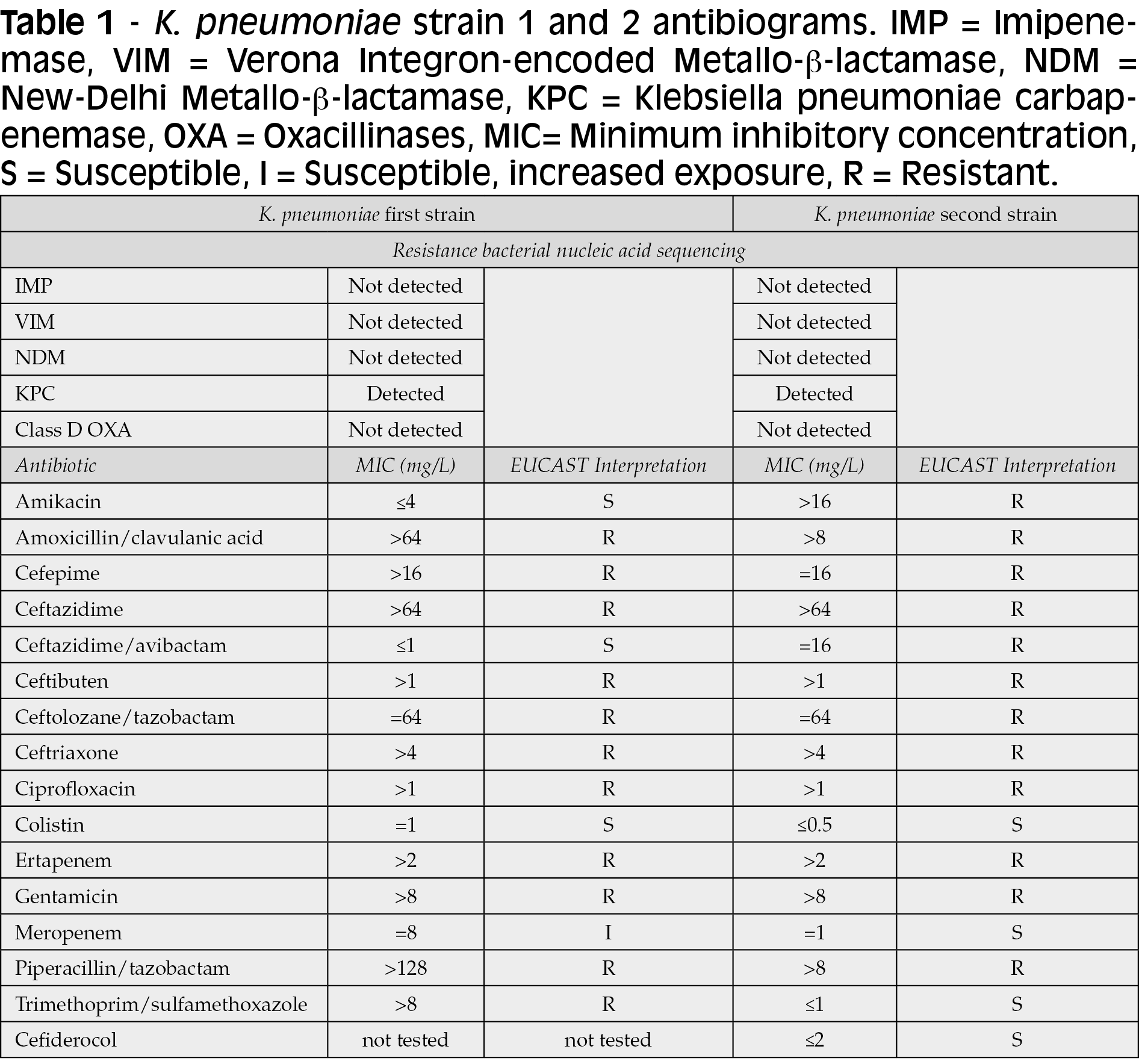
After three days, a CPKP (Table 1) was identified in CSF culture. Antibiotics susceptibility tests were performed using broth microdilution. Clinical breakpoint and susceptibility interpretations were based on EUCAST Jan 1, 2022 criteria. Carbapenem resistance was identified with real-time PCR Cepheid Genexpert®. Blood culture was negative.
Following the microbiological result, linezolid was stopped.
Routinely CSF examinations were performed every four days, and initial remission of biochemical alterations was observed. However, after 15 days of therapy, a new CSF worsening was noted with lowering glucose levels and increasing protein levels along with identification of rising cell counts. Surprisingly, CPKP with a new resistant phenotype was isolated from CSF, showing CZA resistance (Table 1). FDC susceptibility was tested with broth microdiluition.
According to these results, CZA was stopped, and FDC was prescribed at the dosage of 2 g every 6 hours, with an extended infusion of 3 hours. FOS was continued for a few days and then substituted with trimethoprim/sulfamethoxazole (TMP/SMX) at the dosage of 320/1600 mg (15 mg/kg of trimethoprim) every 6 hours. Finally, EVD was replaced.
After five days of treatment, some fungal hyphae were found microscopically from a new CSF sample of the patient. Because of this new microbiological finding, 5 mg/kg/day of liposomal amphotericin B was added to the antimicrobial therapy. Beta-D-glucan was therefore checked on serum and CSF with negative result on both, leading to discontinuation of antifungal therapy.
After one week of FDC plus TMP/SMX, CSF culture was negative and CSF parameters normalised. Therapy continued for another 14 days, with notable progressive improvement of clinical and neurological condition of the patient. Later the EVD was replaced with a ventriculoperitoneal shunt.
Therapeutic timeline is summarised in Figure 1.
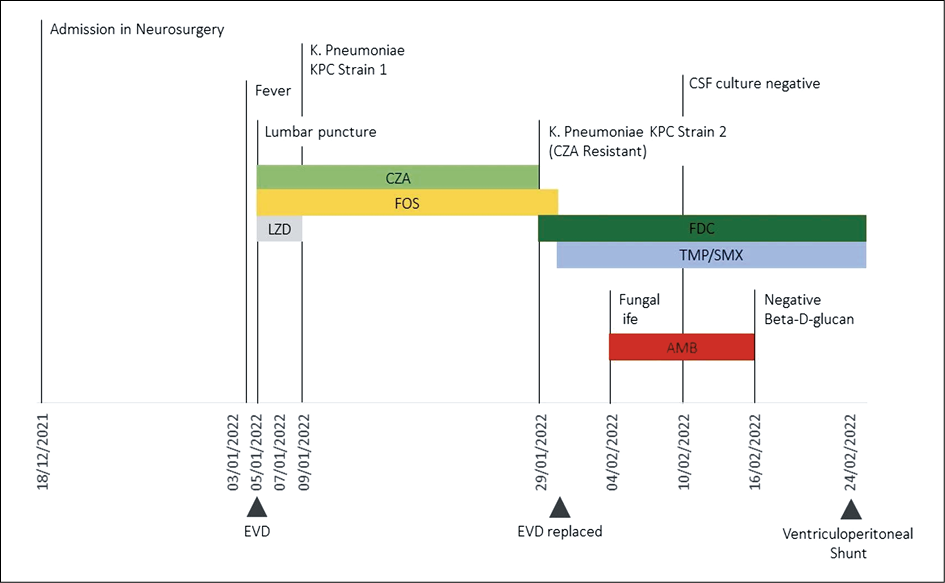
Figure 1 - Therapeutic timeline. EVD=external ventricular drainage.
Notes: CZA=ceftazidime/avibactam, FOS: fosfomycin, LZD=linezolid, FDC=cefiderocol, TMP/SMX=trimethoprim/sulfamethoxazole, AMB= liposomal amphotericin B.
DISCUSSION
Drug-resistant Enterobacterales, particularly K. pneumoniae, represent a public threat worldwide. In settings such as hospitals or health care facilities, increasing rates of antimicrobial resistance limit the actual treatment options [6, 7].
FDC is a new broad-spectrum siderophore cephalosporin against Gram-negative bacteria, including carbapenem-resistant pathogens [8]. Robust preclinical and clinical trials have established the in vivo efficacy of FDC in urinary tract infections, acute pyelonephritis and nosocomial pneumonia [9]. FDC demonstrates primarily urinary excretion and a half-life of 2-3 hours. It presents a protein binding of 58% in human plasma and, as with other β-lactam drugs, FDC is a time-dependent drug [10]. In difficult penetration sites, FDC has shown good outcomes. For example, it is worth of attention that in epithelial lining fluid of lung tissue in critically ill patients with pneumonia, FDC concentrations with minimum inhibitory concentration (MIC) of ≤4 mg/L were sufficient to treat Gram-negative bacterial infections [11]. But, in other human body sites, such as in the CNS, data on FDC penetration are lacking.
In rat models, Takemura et al. demonstrated that FDC penetration rates were similar to other antibiotics, including piperacillin, cefepime and meropenem. Furthermore, in strongly inflamed meninges, FDC penetration was increased 3-fold [12].
In studies with in vivo scenarios, FDC was used to treat multi-drug resistant (MDR) bacterial infections of the CNS in only two cases. FDC was successfully used in association with intravenous and intrathecal colistin in one case report of nosocomial neurosurgical meningitis due to extensively drug-resistant (XDR) P. aeruginosa. Therapeutic drug monitoring (TDM) demonstrated a good CSF concentration. However, clinical success was achieved with lower dosages than recommended (started at 1 g every 8 hours and later increased to 1.5 g every 8 hours) [13]. In another real-life case study, post-craniotomy meningitis caused by resistant P. aeruginosa was treated with FDC. CSF and plasmatic TDM were measured with evidence of good drug concentration. Authors concluded that high-dose FDC (2 g every 6 hours, in extended infusion of 3 hours) could adequately penetrate the CNS [14].
To our knowledge, the described case is the first successful treatment using high-dose FDC in CPKP post-surgical meningitis. Interestingly, the progressive selective pressure of CAZ treatment over fifteen days determined the selection of a resistant strain. Notably, FDC was initially used along with FOS and linezolid and then with TMP/SMX with a progressive improvement of the CSF laboratory findings and the sterilisation of the CSF culture. Data related to combination therapy with FDC is not considered robust at this time, although FDC and FOS combinations have already been described [14]. New therapeutic approaches, such as combination therapies including FDC, should be studied for difficult-to-treat infections.
Our report has some limitations. Firstly, FDC therapeutic drug monitoring (TDM) was not conducted. TDM could be helpful in determining FDC concentrations and its better use in critically ill patients. In addition, in difficult-to-penetrate sites, such as CNS, TDM can allow a more rational use of new drugs for MDR bacterial infections.
Secondly, using a combination therapy did not allow us to accurately assess the role of FDC as a valid alternative in MDR bacterial meningitis. Nevertheless, the severe patient conditions and the insufficient literature data drove clinicians to enhance therapy to increase patient’s chances of recovery.
In conclusion, more data is necessary to better characterise FDC’s role in Gram-negative MDR neurological infections. This real-life experience suggests that FDC could be a valid alternative in this scenario.
Funding
The authors did not receive support from any organisation for the submitted work
Conflicts of interest/competing interests
None
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by NG, FC and AW. The first draft of the manuscript was written by NG and FC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Consent to participate
Informed consent was obtained by the patient.
References
[1] Delarampour A., Ghalehnoo Z.R., Khademi F., Hamid Vaez H. Antibiotic resistance patterns and prevalence of class I, II and III Integrons among clinical isolates of Klebsiella pneumoniae. Infez Med. 2020; 1, 64-69.
[2] Del Prete R., Ronga L., Addati G., et al. Trends in Klebsiella pneumoniae strains isolated from the bloodstream in a teaching hospital in southern Italy. Infez Med. 2019; 1, 17-25.
[3] Esposito S., De Simone G. Update on the main MDR pathogens: prevalence and treatment options. Infez Med. 2017; 4, 301-310.
[4] Gaibani P., Giani T., Bovo F., et al. Resistance to Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam in Gram-negative MDR bacilli: molecular mechanisms and susceptibility testing. Antibiotics. 2022; 11 (5), 628. doi: 10.3390/antibiotics11050628.
[5] Wang C., Yang D., Wang Y., Ni W., et al. Cefiderocol for the Treatment of Multidrug-Resistant Gram-Negative bacteria: a systematic review of currently available evidence. Front Pharmacol. 2022; 13, 896971. doi:10.3389/fphar.2022.896971.
[6] Lasko M.J., Nicolau D.P. Carbapenem-Resistant Enterobacterales: considerations for treatment in the era of new antimicrobials and evolving enzymology. Curr Infect Dis Rep. 2020; 22 (3), 6. https://doi.org/10.1007/s11908-020-0716-3
[7] Geremia N., Prinčič E., De Vito A., et al A rare case of Raoultella planticola spondylodiscitis in an HIV, former drug user, and glucose-6-phosphate dehydrogenase deficiency patient Infect Dis Trop Med. 2020; 6, e643. 10.32113/idtm_20207_643
[8] Aoki T., Yoshizawa H., Yamawaki K., et al. Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other Gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship. Eur. J. Med. Chem. 2018; 155, 847-868. https://doi.org/10.1016/j.ejmech.2018.06.014
[9] Bilal M., El Tabei L., Büsker S., et al. Clinical pharmacokinetics and pharmacodynamics of Cefiderocol. Clin Pharmacokinet. 2021; 60 (12), 1495-1508. https://doi.org/10.1007/s40262-021-01063-5
[10] Katsube T., Echols R., Wajima T. Pharmacokinetic and pharmacodynamic profiles of Cefiderocol, a novel siderophore cephalosporin. Clin Infect Dis. 2019; 69 (Suppl. 7), S552-S558.
[11] Katsube T., Nicolau, D.P., Rodvold K.A., et al. Intrapulmonary pharmacokinetic profile of cefiderocol in mechanically ventilated patients with pneumonia. J Antimicrob Chemother. 2021; 76 (11), 2902-2905. https://doi.org/10.1093/jac/dkab280
[12] Takemura M., Kanazawa S., Kohira N., et al. Evaluation of penetration of Cefiderocol into cerebrospinal fluid using a rat meningitis model. Open Forum Infect Dis. 2021; 645. https://doi.org/10.1093/ofid/ofab466.1300
[13] Stevenson D.R., Cherian, B.P., Kinzig M., et al. P44 Nosocomial neurosurgical meningitis due to XDR Pseudomonas aeruginosa: clinical experience with cefiderocol. JAC Antimicrob Resist. 2022 dlac004.043. https://doi.org/10.1093/jacamr/dlac004.043
[14] Meschiari M., Volpi S., Faltoni M., et al. Real-life experience with compassionate use of cefiderocol for difficult-to-treat resistant Pseudomonas aeruginosa (DTR-P) infections. JAC Antimicrob Resist. 2021; 3 (4), dlab188. https://doi.org/10.1093/jacamr/dlab188