Le Infezioni in Medicina, n. 3, 446-453, 2022
doi: 10.53854/liim-3003-14
ORIGINAL ARTICLES
Influenza-associated severe acute respiratory infections among children under five years old in Morocco, September 2017 to March 2019
Zakia Regragui1,2, Abderrahman Bimouhen1, Fatima El Falaki1, Hassan Ihazmad1, Samira Benkerroum1, Soumia Triki3, Imad Cherkaoui4, Chafiq Mahraoui6, Abdelkarim Filali-Maltouf2, Leila Medraoui2, Hicham Oumzil1,5
1.National Influenza Center, Virology Department, National Institute of Hygiene, RABAT, Morocco;
2Equipe de Microbiologie et Biologie Moléculaire (EMBM), Biology Department, Mohamed V University of RABAT, Morocco;
3World Health Organization, Country Office. Rabat, Morocco;
4Directorate of Epidemiology and Disease Control, Ministry of Health, Rabat, Morocco;
5Pedagogy and Research Unit of Microbiology, and Genomic Center of Human Pathologies, School of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco;
6Hôpital d’Enfants, Centre Hospitalier Universitaire, Rabat, Morocco.
Article received 23 March 2022, accepted 2 July 2022
Corresponding author
Zakia Regragui
E-mail: zakia.regragui@yahoo.com
SummaRY
The main aim of this research is to investigate the trend of influenza infection among children under 5 years with severe acute respiratory infections (SARI) as well as those who suffer from a high burden of disease.
This research is based on a survey conducted from September 2017 to March 2019. During this period nasopharyngeal swabs were collected in a group of 942 children under 5 years with SARI, admitted in pediatric services of 8 sentinel hospitals.
The virological surveillance of influenza was carried out at the National influenza Center, located in the National Institute of Hygiene, using a Reverse transcription polymerase chain reaction (qRt-PCR) monoplex assay developed by the Centers for Disease Control and Prevention (CDC; Atlanta, GA).
The median age of participants was 11 months, and 40% of them were female. A total of 112 samples were reported positive yielding a frequency of 11.88% (112/942). Among all the influenza confirmed cases, 68.75% (77/112), 15.17% (17/112), 16.04% (18/112) were subtyped as influenza AH1N1pdm09, AH3N2 and influenza B respectively. Meanwhile, the proportion of patients admitted at the intensive care unit was 5,35% (6/112). Out of which 83.33% (5/6) were AH1N1pdm09 and it was reported that just 1.78% (2/112) of the positive cases were vaccinated.
The study confirms that influenza affects greatly children with SARI. Thus, the need for influenza vaccines is highly recommended for children under 5 years. Moreover, our findings highlight that influenza virus is not the only cause of SARI among this group of children. Accordingly, special attention should be paid to the non-flu respiratory viruses.
Ke words: influenza, SARI, children.
INTRODUCTION
It is proved that Influenza virus is a serious human pathogen that causes significant morbidity and mortality. Overall, it is annually reported that winter epidemics caused by these viruses affect the whole population worldwide. Consequently, they lead to some economic crises and to severe diseases and mortality [1]. It is further estimated that about 9% of the world population is subject to these seasonal epidemics each year, which are reported to be responsible for about 3 to 5 million cases of serious diseases and 650,000 deaths yearly. Noticeably, it is detected that these cases are associated with respiratory illnesses due to seasonal influenza [2, 3].
The World Health Organization (WHO) estimates that 20 to 30% of children are infected with the influenza virus each year, causing 1 to 2 million cases of SARI and up to 100,000 deaths per year [4]. Although several studies conducted in Morocco suggest that the burden of influenza is significant, there is still uncertainty about the etiology of hospitalizations associated with respiratory diseases in children under 5 years old [5].
Children are likely to be infected twice to three times more frequently than adults [6]. In addition, young children, especially those under the age of six months, are at a higher risk of serious illnesses, hospitalization and death caused by influenza virus than older children [7]. Furthermore, children under 5 years old play a critical role in the transmission of influenza in the community [8]. The most prevalent consequences of pediatric influenza are bronchitis, bronchiolitis, pneumonia, acute otitis media and seizures or febrile convulsions [9, 10]. Clinical features are non-specific as they are commonly reported also for other respiratory pathogen infections which may have a potential impact on patient management in case of a large co-circulation of all these viruses [11]. On the other hand, the lack of sufficient virological and epidemiological surveillance lead to a wide spread of antibiotic resistance because of the abuse and inappropriate use of antibiotics, with negative impacts on the health of individuals as well as on the economy [12].
Accordingly, it is estimated that influenza surveillance is essential to monitor and control influenza infections, to assess the effectiveness of influenza vaccine and to guide clinicians to employ the appropriate therapeutic management of patients [13].
PATIENTS AND METHODS
Influenza surveillance system
The National Influenza Center (NIC), which has been part of the WHO network since 2000, is the one responsible for conducting the virological surveillance of influenza and other respiratory viruses that are the cause of respiratory infections in Morocco. Its main concern is to ensure the identification of antigenic and genetic characterization of influenza viruses responsible for annual epidemics. This is done in order to assess the adequacy of the vaccine composition with circulating influenza viruses as well as the evolution of natural resistance to antivirals. Since 1996 the laboratory has also provided technical support for epidemiological surveillance through a network of voluntary doctors from the private sector including: general practitioners, pediatricians and pneumo-phthisiologists. This surveillance is based on the weekly recordings of influenza-like illness (ILI) and SARI which are diagnosed by doctors.
The epidemiological surveillance of influenza is also carried by a network of health centers and hospitals selected from 8 Moroccan regions, namely: Rabat-Sale-Kenitra, Fes-Meknes, Souss-Massa, Beni Mellal-Khenifra, Tanger-Tetouan, Marrakech-Safi, Laayoune-Saguia Al Hamra and Oriental. This network, headed by the Department of Epidemiology and Disease Control since 2004, estimates the weekly number of consulting patients for ILI and SARI as well as the virological testing of samples within the platform of the NIC throughout the year.
Study population
The study was conducted at the NIC at the National Institute of Hygiene in Morocco. The samples tested were collected from 8 sentinel sites distributed throughout the country. It targeted a group of children under 5 years who were admitted to pediatric services during September 2018 to March 2019 period. The study involved all patients fulfilling the WHO definition for SARI : acute respiratory infection (ARI) with a history of fever, or fever measured ≥38°C, cough with onset of symptoms within the last 10 days that requires hospitalization [14]. The samples were then stored in an appropriate viral transport medium, at +4°C and sent to the NIC with a patient investigation form filled duly by a clinician, within a period of 48 hours maximum according to the current biosafety measures.
Extraction of nucleic acids
Viral nucleic acids were automatically extracted from 400ul of respiratory specimens by using a High Pure Viral Nucleic Acid Kit and iPrep instrument. This was done in accordance with the manufacturer’s recommendation (Lifetechnologies, Carlsbad, USA). Viral RNA was eluted in a volume of 100 μl and processed immediately or stored at -80°C before testing.
Detection of Influenza virus by qRT-PCR
All samples were tested for the presence of influenza virus. The detection and subtyping of Influenza virus was performed by qRt-PCR and the SuperScript III Platinum® One-Step qRT-PCR System (Invitrogen, Scientific ThermoFisher, USA). The samples were first screened for the detection of influenza A and B viruses. Positive Samples for influenza A virus were then tested for AH1N1pdm09 and AH3N2. The genetic lineage Yamagata and Victoria of detected influenza B viruses were also tested by qRt-PCR. Primers, probes and positive controls were provided by International Reagent Resource (IRR, USA). The amplification was performed on the Applied Biosystems 7500 Fast platform using a qRt-PCR according to the CDC protocol (CDC; Atlanta, GA) [15]: reverse transcription at 50°C for 30 min, inactivation of the Taq inhibitor at 95°C for 2 min, then 45 cycles of denaturation at 95°C for 15 s and annealing/amplification at 55°C for 30 s. Positive samples had a cycle threshold value (Ct) <38.
Statistical analysis
To collect data, clinicians duly filled a standard surveillance form. Clinical, virological and demographic data were then recorded using the laboratory information system (Kalisil) which generates an excel file. Analysis of the results was performed by Epi-info version 7.1 software developed by the CDC (CDC; Atlanta, USA). The Pearson Chi-square or Fisher exact test estimated group comparisons as appropriate. Meanwhile, P-values for interactions below 0.05 were considered statistically significant. Proportions, means and all statistical analyses were performed using the same software.
RESULTS
Demographic characteristics
During the study period of September 2017 to March 2019, 942 nasopharyngeal swabs were collected from children under 5 years old, who were admitted in sentinel hospitals from 8 regions distributed nationwide. The notification forms were then reviewed and 81.5% (768/942) of the children fulfilled the WHO’s case definition of SARI. The sex ratio (male/female) was 1.45 (559/383) and the age of the enrolled patients ranged from 0 days to 5 years (median age 11 months) whereas children under 6 months and between 6 and 23 months represented 42.56% (401/942) and 41.93% (395/942) respectively out of the total number of SARI cases. On the otherhand, children over 24 months represented the lowest age group with a proportion of 15.49% (146/942). Among the 8 sentinel hospital regions, the largest number of specimens collected was in Rabat-Sale-Kenitra (281/942) with a proportion of 29.83%. Specimens were collected throughout all study period but the largest number was collected during the first quarter which corresponds to the cold and wet season in Morocco (January to the end of March) (638/942) with a frequency of 67.8% (Table 1).

Detection of Influenza virus
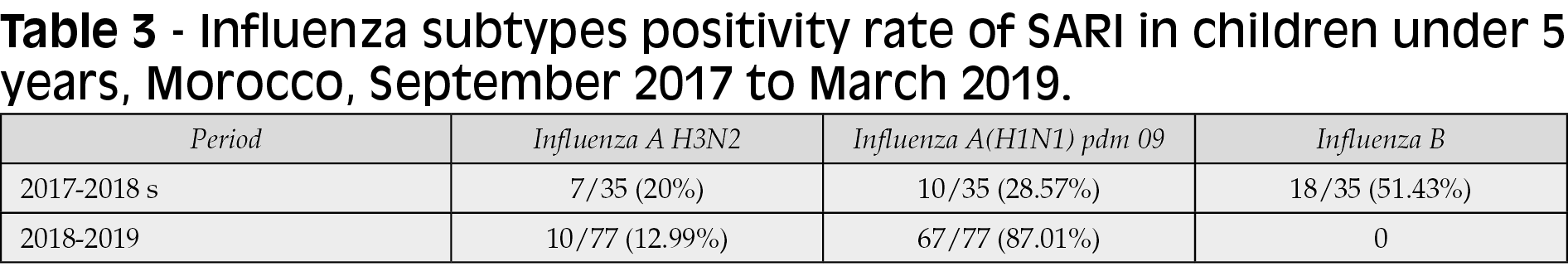
Out of 942 SARI specimens tested among children under 5 years old, a total of 112 samples were reported positive yielding a frequency of 11.89% (112/942) (Table 2). Among all influenza confirmed cases, 68.75% (77/112), 15.17% (17/112), 16.04% (18/112) were subtyped as influenza AH1N1pdm09, AH3N2 and B respectively. Out of which 94.44% (17/18) were subtyped as influenza B/yamagata (Table 3).
It is reported that the detection rate for influenza was higher among the children between 6 and 23 months (47.32% ; 53/112), followed by those aged between 2 and 5 years (30.35% ; 34/112) and then children under 6 months (22.32% ; 25/112) (Table 1).
Clinical manifestations
The common clinical manifestations in the positive cases among our group of study were the caught (98.20%; 109/112). The onset of symptoms in patients for influenza within 10 days of admission was in 98.28% (110/112), and the fever was found in 98.20% (109/112). Consequently, 12.5% (14/112) of the cases were admitted in the pediatric unit, 12.5% (14/112) in pediatric emergency unit whereas 5.35% (6/112) at the intensive care unit of which 83.33% (5/6) were subtyped as influenza AH1N1pdm09 and all of them was unvaccinated. Based on the patient data form, only 1.78% (2/112) of the positive cases were vaccinated while 89.21% (110/112) were unvaccinated (Table 1).
Influenza seasonal distribution
It was observed that the influenza virus was detected mainly from November to April during 2017-2018 and 2018-2019 which correspond to the cold and wet season in Morocco. However, it was noticed that viral activity peaked in January 2017-2018 (14/35; 40%) and in February (63/77; 81.81%) 2018-2019. Positivity rate was noticed during the 2018-2019 period (77/112; 68.75%) as compared to 2017-2018 period (35/112; 31.25%) (Figure 1 and Table 2).
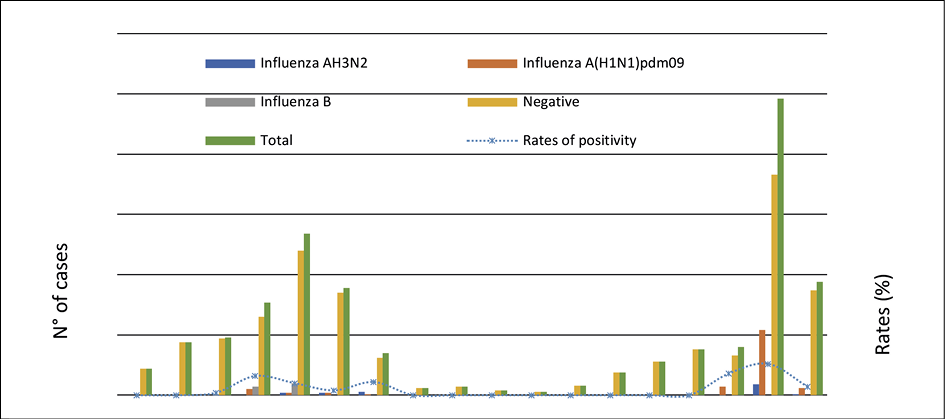
Figure 1 - Rates of samples tested and proportion of positivity for Influenza of SARI in children under 5 years by months, Morocco, September 2017-March 2019.

The analysis of the influenza virus characteristics, based on the seasonal circulation, showed that Influenza B virus was the most frequent source of infection during the 2017-2018 influenza season (18/35; 51.43%), followed by influenza AH1N1pdm09 (10/35; 28.57%) and AH3N2 (7/35; 20%). Whereas, the AH1N1pdm09 and AH3N2 subtypes co-circulated during the 2018-2019 season, with a predominance of the AH1N1pdm09 (67/77; 87.01%) followed by the AH3N2 (10/77; 12.99%) (Table 3).
DISCUSSION
It is estimated that Influenza remains a major cause of illness and death worldwide as well as a significant economic burden [6]. While most people recover fully from influenza infection, the consequences of potential complications in different age groups, especially in children under 5 years, remain uncertain within the Moroccan context in particular.
On the basis of the data collected from the sentinel influenza surveillance within the framework of the NIC, it is reported that influenza virus was one of the causes of SARI during 2017-2018 and 2018-2019 seasons in Morocco. This was clearly noticed in group of children under 5 years who represent a high risk group of influenza complications, resulting in serious diseases, increased hospitalizations, and mortality [16]. The positivity rate in our study group was 11.88%, where in the predominant circulating subtype was the influenza B virus (18/35; 51,43%) during 2017-2018 season. This agrees with overall patterns of influenza circulation reported in Northern Africa and Western Asia by the WHO in the same period in which all subtypes of influenza were detected, with the predominant strain varying by country [17]. Influenza B viruses were the most common in Azerbaijan and Georgia, whereas influenza A and B circulated in almost similar quantities in Armenia, Cyprus and Israel. It was also observed that Influenza AH3N2 viruses were in circulation but in lower proportions. The Yamagata lineage was found in the majority of influenza B virus in circulation during the same period [17].
However, in accordance with our study, all subtypes of influenza A viruses co-circulated with a predominance of AH1N1pdm09 (67/77; 87,01%) during 2018-2019 period. This matches what the WHO has reported in Northern Africa and western Asia in the same period, with a predominance of influenza A viruses over all influenza viruses detected [18]. The prevalent subtype varied by country. In Algeria and Egypt, influenza AH3N2 viruses predominated, while AH1N1pdm09 was reported to be more frequent in Western Asia (especially in Armenia, Georgia, Kuwait, and Qatar). Influenza AH3N2 spread throughout Western Asia, though in low numbers. In Iraq, Israel, and Turkey it was estimated to be in higher proportions in comparison with influenza AH1N1pdm09 [18] and were therefore considered as an exception. In comparison to some strains of influenza A, influenza B is thought to be a milder virus [19]. However, the mortality rates associated with influenza B infection is significantly higher compared to influenza A in children under 16 years old [20].
During the first quarter of this study period, influenza virus circulation in Morocco among children under 5 years with SARI was clearly noticeable and it peaked in January of 2017-2018 flu season, and in February of 2018-2019 flu season. This is similar to the influenza seasonality observed in Algeria, Azerbaijan, Cyprus, Kuwait and Tunisia, where influenza activity did not increase until early or late January. However, in other countries it increased earlier (Bahrain, Egypt, Kuwait, Oman and Qatar) [17, 18].
It is reported that the influenza-associated SARI rate in our study population remains lower in comparison with other studies which estimated that among children under 5 years of age, there have been 5 million influenza virus episodes and approximately 10 million cases of influenza-associated SARI [7]. This low rate in Morocco is probably not due to the improved influenza vaccine management in this age group. In fact, according to our study, the vaccination coverage remains very low 1.78% (2/112). Therefore, annual influenza vaccination is recommended for young children, especially for those aged ≥6 months to reduce the risk of severe complications [21]. It is therefore observed that the admission in the Intensive Care Unit (ICU) has been reported in 5.35% of positive cases in children under 5 years and all of them were not vaccinated. It follows that influenza vaccination remains the best method of prevention against influenza infection and its associated complications [22]. Routine annual influenza vaccination for all children older than 6 months with no contraindications have been also recommended by the CDC and the CDC Advisory Committee on Immunization Practices (ACIP) since 2010 [21]. In fact, several factors suggest that the influenza vaccine is a possible effective measure to control antibiotic use and also to reduce antibiotic resistance by reducing significantly outpatient visits for influenza-associated ARI for which antibiotics are routinely prescribed [21, 14]. It is then reported that improved influenza vaccine coverage and effectiveness, diagnosis and recognition along with efforts to limit antibiotic use are critical measures for reducing antibiotic prescriptions and thereby mitigating the growth in antibiotic resistance [23]. Hence the need for a public awareness program is strongly recommended in Morocco to increase the vaccination coverage each year, especially among the high-risk groups.
The co-circulation of other respiratory viruses during influenza seasons, particularly respiratory syncytial virus (RSV), is a significant impediment to estimating the number of influenza cases associated to SARI in children under 5 years in Morocco [24, 25].
The epidemiology is deeply changed during the two years following the present investigation due to COVID 19 pandemic [26]. Indeed, some authors reported a significant sudden decline in the prevalence of all the other respiratory viruses, especially those typically isolated during the winter season, such as RSV and Influenza virus compared to the previous years [27]. This decrease can be attributable to the public health measures and non-pharmacological interventions (NPIs) adopted such as social distancing, facemask wearing, adequate hand hygiene, surface disinfection and ventilation of indoor spaces [28].
Overall, this study is not an exhaustive one. It has encountered some limitations as far as the unavailability of epidemiological and clinical data on SARI in Morocco are concerned. Thus, this makes difficult to implement evidence-based management and prevention strategies. Accordingly much effort should be made to develop research on all areas to obtain a holistic approach to better understand the determinants of SARI in Morocco and to establish adequate control measures.
In conclusion, overall, our findings provided evidence that influenza is a significant burden in children with SARI. Furthermore, it was shown that influenza virus is not the only source of infection of SARI in children under 5 years. In fact, much attention and care should be paid to the non-flu respiratory virus and bacterial pathogens.
It is also reported that the pandemic invasion of the new SARS-CoV-2 virus in 2019 has been accompanied by the disappearance of existing circulating strains of Influenza and other respiratory viruses. This is due to the wearing of face masks, social distancing and hand hygiene [29]. These NPIs has positively affected the transmission of Influenza viruses and provided a possibility to make better choices in the future of adapting a strategy to fight the spread of the respiratory viruses.
In order to better understand the epidemiology, etiology, antiviral susceptibility patterns, and effectiveness of the preventive and therapeutic interventions in place against pediatric SARI in Morocco, more effort should be put into enhancing sufficient surveillance programs.
Ethics approval and consent to participate
The data at hand were obtained from the influenza sentinel surveillance system, which is approved by the Ministry of Health for national surveillance of respiratory diseases. Accordingly, patient confidentiality is respected. Thus, the study does not require formal ethical review. So, verbal consent was obtained from parents prior to sample taking.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests
Funding
This work was supported by the Centers for Disease Control and Prevention (CDC), grant n° 5U51CI000469 and WHO in frame work of Pandemic Influenza Preparedness (PIP).
Authors’ contributions
HO, ZR, FE, LM and AFM conceived and designed the study. ST, IC and HO provided data and materiel. AB, HI, FE, SB and IC contributed on collection and processing. ZR and HO performed the analysis. ZR wrote the paper. HO, LM and AFM reviewed the draft. All authors read and approved the final manuscript.
Acknowledgements
We would like to express our sincere gratitude to the medical doctors, nurses and laboratory technicians in the public network for their kind support on clinical sample collection.
REFERENCES
[1] Uyeki TM, Bernstein HH, Bradley JS et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis. 2019; 68 (6): e1-47.
[2] Horm SV, Mardy S, Rith S et al. Epidemiological and Virological Characteristics of Influenza Viruses Circulating in Cambodia from 2009 to 2011. Krammer F, editor. PLoS ONE. 2014; 9 (10): e110713.
[3] Krammer F, Smith GJD, Fouchier RAM et al. Influenza. Nat Rev Dis Primer. 2018; 4 (1): 3.
[4] Influenza (Seasonal) [Internet]. [cited 2021 Nov 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
[5] Barakat A, Ihazmad H, Benkaroum S et al. Influenza Surveillance among Outpatients and Inpatients in Morocco, 1996–2009. PLoS OneE. 2011; 6 (9): e24579.
[6] Kurskaya O, Ryabichenko T, Leonova N et al. Viral etiology of acute respiratory infections in hospitalized children in Novosibirsk City, Russia (2013-2017). PLoS One. 2018;13(9):e0200117.
[7] Nair H, Brooks WA, Katz M et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 20113; 378 (9807): 1917-1930.
[8] Wang X, Li Y, O’Brien KL, Madhi SA et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. 2020; 8 (4): e497-510.
[9] Poehling KA, Edwards KM, Griffin MR et al. The Burden of Influenza in Young Children, 2004–2009. Pediatrics. 2013; 131 (2): 207-216.
[10] Brooks WA, Goswami D, Rahman M et al. Influenza is a major contributor to childhood pneumonia in a tropical developing country. Pediatr Infect Dis J. 2010; 29 (3): 216-221.
[11] Bouzid D, Mullaert J, Le Hingrat Q et al. Characteristics associated with COVID-19 or other respiratory viruses’ infections at a single-center emergency department. PloS One. 2020; 15 (12): e0243261.
[12] Jroundi I, Benmessaoud R, Mahraoui C et al. Antibiotic usage prior and during hospitalization for clinical severe pneumonia in children under five years of age in Rabat, Morocco. Antibiotics. 2013; 2 (4): 450-464.
[13] Bansal A, Trieu MC, Mohn KGI, Cox RJ. Safety, Immunogenicity, Efficacy and Effectiveness of inactivated influenza vaccines in healthy pregnant women and children under 5 years: an evidence-based clinical review. Front Immunol. 2021; 12: 3926.
[14] Fitzner J, Qasmieh S, Mounts AW et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018; 96 (2): 122-128.
[15] CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf [Internet]. [cited 2021 Nov 29]. Available from: https://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf
[16] Heikkinen T. Respiratory viruses and children. J Infect. 2016; 72, S29-33.
[17] Hammond A, Laurenson-Schafer H, Marsland M, Besselaar T, Fitzner J, Vandemaele K. Bilan de la saison grippale 2017-2018 dans l’hémisphère Nord. 2018; 34, 16.
[18] World Health Organization = Organisation mondiale de la anté. Review of the 2018-2019 influenza season in the northern hemisphere – Bilan de la saison grippale 2018-2019 dans l’hémisphère Nord. Wkly Epidemiol Rec Relevé Épidémiologique Hebd. 2019; 94 (32), 345-363.
[19] Sharma L, Rebaza A, Cruz CSD. When “B” becomes “A”: the emerging threat of influenza B virus. Eur Respir J [Internet]. 2019 Aug 1 [cited 2022 Jun 10];54(2). Available from: https://erj.ersjournals.com/content/54/2/1901325
[20] Tran D, Vaudry W, Moore D, Bettinger JA et al. Hospitalization for Influenza A Versus B. Pediatrics. 2016; 138 (3), e20154643.
[21] Grohskopf LA, Alyanak E, Broder KR et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2020-21 Influenza Season. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2020; 69 (8), 1-24.
[22] CDC. Children & Influenza (Flu) [Internet]. Centers for Disease Control and Prevention. 2021 [cited 2021 Nov 30]. Available from: https://www.cdc.gov/flu/highrisk/children.htm
[23] Smith ER, Fry AM, Hicks LA et al. Reducing Antibiotic Use in Ambulatory Care Through Influenza Vaccination. Clin Infect Dis. 2020; 71 (11), e726-34.
[24] Bimouhen A, El Falaki F, Ihazmad H, Regragui Z, Benkerroum S, Barakat A. Circulation of respiratory syncytial virus in Morocco during 2014-2016: Findings from a sentinel-based virological surveillance system for influenza. EMHJ-East Mediterr Health J. 2016; 22 (7), 482-489.
[25] Jroundi I, Mahraoui C, Benmessaoud R, Moraleda C, Benjelloun B, Bassat Q. Knowledge gaps on paediatric respiratory infections in Morocco, Northern Africa. Arch Public Health. 2015; 73 (1), 28.
[26] Kim KW, Deveson IW, Pang CNI et al. Respiratory viral co-infections among SARS-CoV-2 cases confirmed by virome capture sequencing. Sci Rep. 2021; 11(1), 3934.
[27] Ippolito G, La Vecchia A, Umbrello G et al. Disappearance of seasonal respiratory viruses in children under two years old during COVID-19 pandemic: a monocentric retrospective study in Milan, Italy. Front Pediatr. 2021; 9, 721005.
[28] Groves HE, Piché-Renaud PP, Peci A et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg Health Am. 2021; 1, 100015.
29] Wu D, Lu J, Liu Y, Zhang Z, Luo L. Positive effects of COVID-19 control measures on influenza prevention. Int J Infect Dis. 2020; 95, 345-346.