Le Infezioni in Medicina, n. 3, 432-439, 2022
doi: 10.53854/liim-3003-12
ORIGINAL ARTICLES
Deoxycholate amphotericin for management of mucormycosis: a retrospective cohort study from South India
Nitin Gupta1,2, Sourabh Srinivas1, Anagha Harikumar1, K Devaraja3, Vishnu Teja Nallapati1, Kavitha Saravu1,2
1Department of Infectious Diseases, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India;
2Manipal Center for Infectious Diseases, Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, Karnataka, India;
3Department of Otorhinolaryngology, Kasturba Medical College and Hospital, Manipal, Manipal Academy of Higher Education, Manipal, Karnataka, India
Article received 25 May 2022, accepted 8 July 2022
Corresponding author
Kavitha Saravu
E-mail: kavithasaravu@gmail.com
SummaRY
Introduction: Liposomal amphotericin use is limited in developing countries due to its extremely high cost and availability. Therefore, the study aimed to evaluate deoxycholate amphotericin B’s utility and adverse effect profile in patients with mucormycosis.
Methodology: This retrospective cohort study from 2019 to 2021 included patients with proven mucormycosis who received deoxycholate amphotericin B for more than or equal to five days and had at least three creatinine values on treatment. Baseline demographic details, risk factors and treatment details of all the patients were recorded. In addition, the details of treatment-related adverse effects and outcomes were ascertained.
Results: Of the 57 included patients, a history of diabetes, COVID-19 and steroid use was present in 49 (86%), 43 (75.4%) and 33 (57.9%) patients, respectively. Isolated rhino-orbital mucormycosis was the most common presentation (n=49, 86%). The median time of follow-up was 48 (30.5-90) days. A total of 8 (14%) patients died during the hospital stay. The median duration of amphotericin treatment was 21 (14-40) days. Thirty-nine patients (68.4%) developed hypokalaemia on treatment, while 27 (47.4%) patients developed hypomagnesaemia. A total of 34 (59.6%) patients developed AKI on treatment. The median day of development of AKI was 6 (4-10) days. The median baseline, highest and final creatinine values were 0.78 (0.59-0.94) mg/dl, 1.27 (0.89-2.16) mg/dl and 0.93 (0.74-1.59) mg/dl respectively. The median percentage change from baseline to highest value and last follow-up value was 45% (0.43%-161%) and 25% (-4.8%-90.1%) respectively. The final creatinine was less than 150% of the baseline in 36 (63.2%) patients.
Conclusion: Deoxycholate amphotericin is an acceptable alternative for treating mucormycosis in resource-constrained settings.
Keywords: Mucormycetes, rhino orbital, acute kidney injury, hypokalaemia, deoxycholate amphotericin B.
INTRODUCTION
Mucormycosis is caused by various species of ubiquitous fungi belonging to the Mucormycetes family [1]. Its incidence in South East Asia is higher owing to the propensity of fungus to favour tropical climates and the high prevalence of DM in these areas [1]. The incidence increased further during the second wave of Coronavirus disease 2019 (COVID-19) due to the inappropriate and indiscriminate use of steroids to treat COVID-19 [2]. The drug of choice for mucormycosis is liposomal amphotericin B [3]. However, its use is limited in developing countries due to its extremely high cost and availability [4]. Deoxycholate amphotericin is cheap but owing to the high incidence of associated nephrotoxicity, its use is not recommended for routine use. Despite this, clinicians in India are forced to use deoxycholate amphotericin for management without feasible alternatives. Therefore, the study aimed to evaluate deoxycholate amphotericin B’s utility and adverse effect profile in patients with mucormycosis.
PATIENTS AND METHODS
This retrospective cohort study was conducted after taking permission from the Institutional Ethical Committe of Kasturba Medical College, Manipal, India.
All patients admitted with a diagnosis of mucormycosis between 2019 and 2021 were screened. Those patients with a confirmed microbiological diagnosis (microscopy or culture positive) and who received deoxycholate amphotericin B were included in this study. Those patients who received amphotericin for less than five days and had less than three creatinine values while on treatment were excluded.
Demographic details such as age, gender, month and year of the presentation were entered in a pre-defined case record form. A diagnosis of COVID-19 at the time or within one year of diagnosis of mucormycosis was recorded in all patients. The time from symptom onset of COVID-19 to symptom onset of mucormycosis was also calculated. The history of use of steroids and the requirement for oxygen during the COVID-19 episode were ascertained from the case records. History of diabetes mellitus (newly diagnosed or otherwise), glycated haemoglobin (HBA1C) and random blood sugar levels at presentation were recorded. History of malignancy and associated febrile neutropenia at presentation was also recorded. All the cases were categorised into rhino orbital mucormycosis (ROM), pulmonary mucormycosis, cutaneous mucormycosis and bone mucormycosis.
The diagnosis of patients with suspected mucormycosis in our centre is usually made using microscopy {potassium hydroxide (KOH) mount or histopathological examination (HPE)} or culture (Sabourad dextrose media) or both. Positivity on microscopy or culture was recorded. The treatment details of all the patients, both medical and surgical, were recorded.
The patients were monitored for development of the common side effects that include Acute Kidney Injury (AKI), hypokalaemia (less than 3.5 mEq/litre) and hypomagnesaemia (less than 1.6 mEq/litre). The minimum levels of potassium and magnesium were noted. AKI was defined as an increase in creatinine from baseline by 150% within seven days or an increase in the absolute value of 0.3 mg/dl within two days (Kidney Disease: Improving Global Outcomes definition) [5]. The day of development of AKI was noted. Creatinine levels on days 0, 2, 4, 6, 8, 10, 12, 14, 30 and 90 on treatment were noted. The percentage change between baseline creatinine and the highest recorded creatinine value on or after treatment was calculated. Similarly, the percentage change between baseline creatinine and the final creatinine value (last available creatinine value of the sequence mentioned above) was also recorded. The outcome of in-hospital stay was categorised as dead or alive on treatment.
Data analysis: Qualitative variables were expressed as percentages whereas quantitative variables were expressed as Mean (± Standard deviation) and Median (Inter-quartile range). The baseline parameters between those who died and those who survived were compared. The Chi-square test was used for qualitative variables while the independent-t-test was used for quantitative variables. A p-value of less than 0.05 was considered significant.
RESULTS
Of 71 patients with mucormycosis admitted during this period, 57 (80.3%) met the inclusion criteria. The mean age of the patients was 49.7±14.8 years. A total of 39 (68.4%) patients were male (Table 1). A total of 49 (86%) patients had DM, eleven (22.4%) of whom were diagnosed with mucormycosis at the time of admission (Table 1). The median random blood sugar level was 157 (102-269) mg/dl at presentation. The mean HBA1c at presentation was 9.9±3.
A total of 43 (75.4%) patients were diagnosed with COVID-19 within a year of diagnosis of mucormycosis (Table 1). Of these 43 patients, six patients (13.9%) were diagnosed with COVID after the onset of mucormycosis symptoms. The median duration from onset of COVID-19 symptoms to the beginning of symptoms for mucormycosis was 20 (10-75) days. Most patients (n=25, 58.2%) developed mucormycosis within 20 days of COVID-19 symptoms. A total of 33 (76.7%) patients received steroids for COVID-19 (Table 1). However, a history of oxygen requirement during COVID admission was present in only 20 (46.5%) patients (Table 1). Patients with COVID-19 who required oxygen therapy were given through either nasal prongs or face masks. Mechanical ventilation was not required for any of the patients. In the fourteen patients where the details were available, methylprednisolone was used in four and dexamethasone in the rest of the ten patients. The median duration of steroid use was 7.5 days (5-10 days). Dexamethasone was used at the dosage of 8 mg once to twice daily while methylprednisolone was used at the dosage of 40 mg once to twice daily
Isolated ROM was the most common presentation (n=49, 86%), followed by isolated pulmonary mucormycosis (n=4, 7%) (Table 1). Of the 51 patients with ROM, all 51 patients had evidence of sinus involvement (maxillary-51, sphenoidal-36, ethmoidal-35, and frontal-27). The palate was involved in 11 patients. Of the patients with ROM (n=53), clinical or radiological evidence of ocular and cerebral involvement was seen in 35 (66%) and 15 (28.3%) patients, respectively.
Of the 57 patients, all were positive by microscopy (KOH or HPE), but only 22 (38.6%) were culture positive (Table 1). Except for eight patients who did not undergo surgical debridement, they all underwent at least one surgical debridement (Table 1). Fourteen patients required two surgical debridements, while one had three debridements. All 57 patients were treated with deoxycholate amphotericin B. The mean dose of amphotericin per day was 56.8±9.6 mg. The median cumulative amphotericin dose was 1470 mg (840-2100). The median duration of amphotericin treatment was 21 (14-40) days.
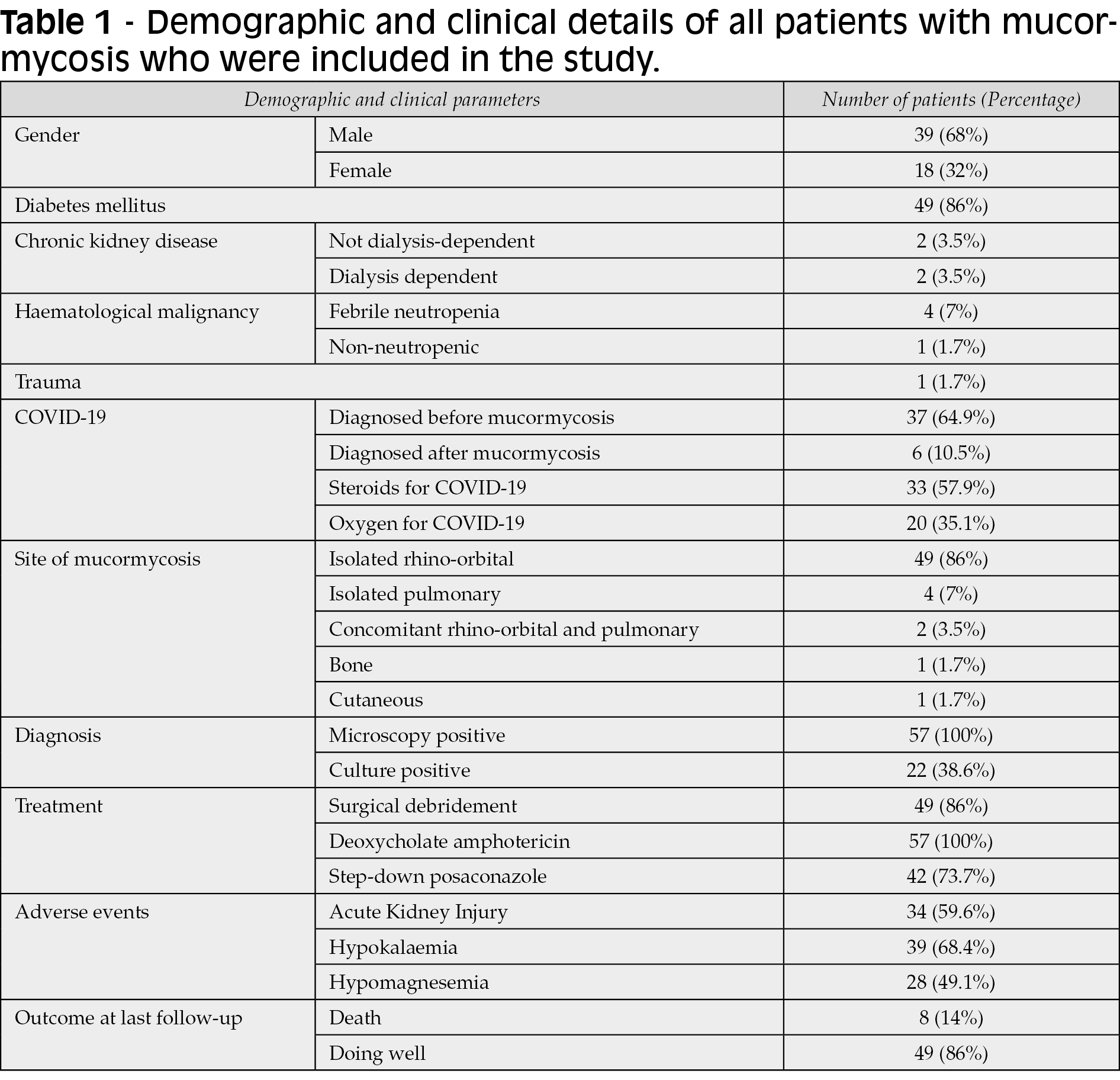
A total of 39 (68.4%) patients developed hypokalaemia on treatment, while 28 (49.1%) patients developed hypomagnesaemia (Table 1). The mean minimum potassium and magnesium levels were 2.6±0.7 and 1.3±0.2, respectively. A total of 34 (59.6%) patients developed AKI on treatment. The median day of development of AKI was 6 (4-10) days. The median baseline, highest and final creatinine values were 0.78 (0.59-0.94) mg/dl, 1.27 (0.89-2.16) mg/dl and 0.93 (0.74-1.59) mg/dl respectively. The median percentage change between baseline and highest creatinine was 45% (0.43%-161%). The median percentage change between baseline and final creatinine was 25% (-4.8%-90.1%). The final creatinine was less than 150% of the baseline in 36 (63%) patients.
The median duration of admission was 17 (8-24.5) days. A total of 8 (14%) patients died during the follow-up period (Table 1). None of the factors was a significant predictor of death on univariate analysis (Table 2). Of the remaining 49 patients who were doing well at discharge, 42 (85.7%) patients were transitioned from intravenous amphotericin to oral posaconazole at discharge. The median duration of posaconazole treatment was 30 (16.5-60) days. The median time of follow-up was 48 (30.5-90) days.
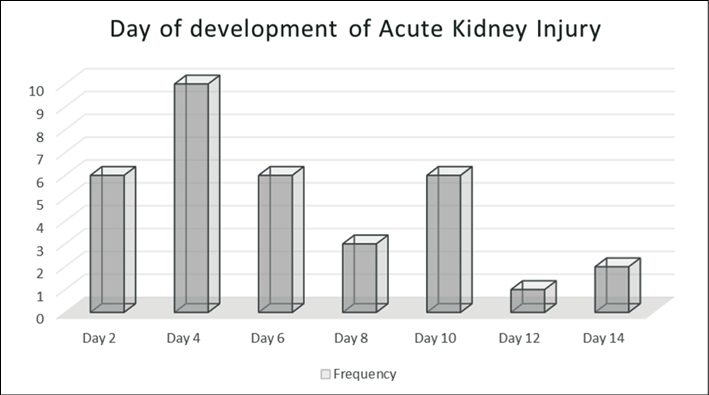
Figure 1 - Number of patients with Acute Kidney Injury on amphotericin and the day of development.
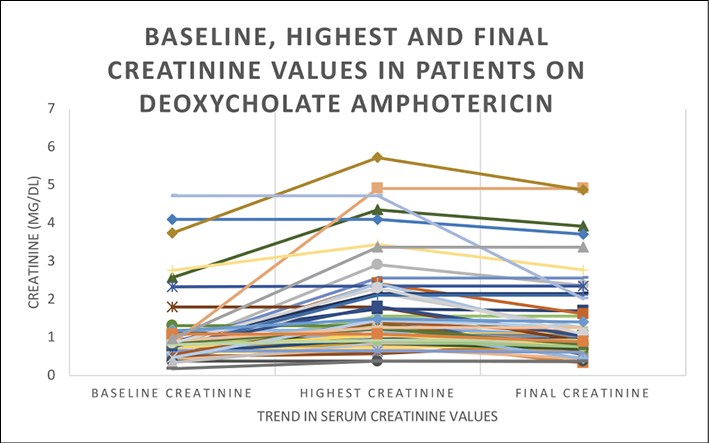
Figure 2 - Baseline, highest and final creatinine values in patients on deoxycholate Amphotericin (n=57).
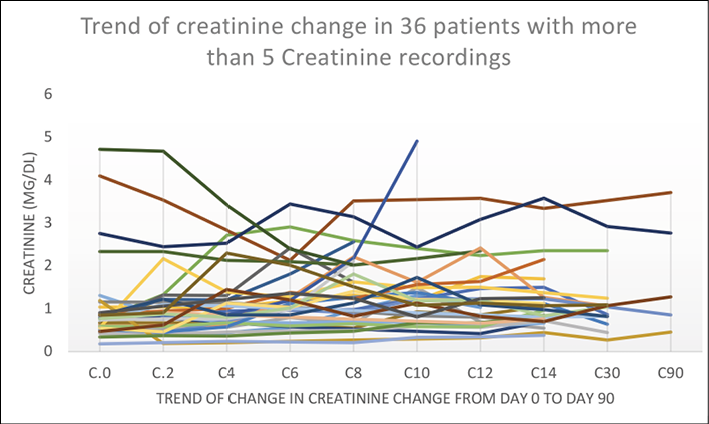
Figure 3 - Trend of creatinine change in those patients with more than five creatinine recordings (n=36).

DISCUSSION
Similar to previous studies, DM was the most common risk factor in patients with mucormycosis in this study [6-8]. The risk is even higher in those with a higher degree of poor sugar control, as evidenced by the mean HBA1C of around 10% in this study. The mean HBA1c in another study in patients with mucormycosis was 10.7% [6]. Hyperglycaemia renders the phagocytic cells dysfunctional, making a patient prone to fungal invasion [9]. Similar to our study, previous studies have shown that DM was first diagnosed at the time of diagnosis of mucormycosis in some cases. Steroid use is another common risk factor for mucormycosis [7]. Besides causing hyperglycemia, its use is also associated with dysregulated immune response. Both DM and steroid use was postulated to be the driving forces that led to an increase in COVID-associated mucormycosis cases [2]. Two-thirds of the patients in our cohort had a recent history of COVD-19. Similar to other studies, the time to onset of post-COVID mucormycosis was 2-3 weeks in most patients [6, 8, 10]. It is interesting to note that more than 30% of the steroid use in the patients of this cohort was inappropriate. Similar findings were observed in other studies [8, 10]. Similar to other studies, ROM followed by pulmonary mucormycosis was the most common type of mucormycosis [7]. In ROM, the infection begins from the sinus and can rapidly spread to involve orbits and the brain or progress towards the palate. It is worthwhile to mention that pulmonary involvement is easier to miss, especially if associated with COVID, as symptoms can be confused with long COVID.
Direct microscopy using KOH mount or histopathological staining is one of the fastest ways to make a reliable diagnosis of mucormycosis [3]. Although the fungus grows in 2-3 days on culture, the sensitivity of fungal culture is poor [3]. The culture was positive in only 39% of the patients in our study. Early suspicion and initiation of appropriate management are paramount. Management of mucormycosis is built on three tenets: control of the underlying disease, aggressive surgical debridement and administration of appropriate antimicrobials [3, 4]. Aggressive debridement of involved tissues is essential to decrease the fungal burden and improve the penetration of antifungals [11]. A total of 86% of the patients in this cohort underwent at least one surgical debridement. Delay in antifungals is associated with an increase in mortality [12]. Therefore, the standard of care is acute administration of antifungals in those with high suspicion of the disease.
The drug of choice for treating mucormycosis is liposomal amphotericin B [3]. The liposomal form is preferred because of its favourable toxicity profile. However, it is costly, and its availability may be restricted in outbreak settings [4]. Isavuconazole and posaconazole are used for step-down therapy after initial treatment with liposomal amphotericin. These azoles can be used as first-line alternatives in patients where liposomal amphotericin B is contraindicated [13, 14]. However, the data on using these azoles for the primary management of mucormycosis is limited. Besides, both of them are expensive as well. Deoxycholate amphotericin B is a cheap alternative but is associated with renal toxicity and hypokalemia. It is, therefore, not recommended by the international guidelines. Due to a lack of alternative option in resource-limited settings, deoxycholate amphotericin has been used in its place despite the limited data. Most of the available literature on deoxycholate amphotericin is from clinical trials involving the treatment of cryptococcal meningitis. However, since the median duration of therapy in this study was longer than most regimens for cryptococcal meningitis, the results of this study may turn out to be helpful in evaluating the role of deoxycholate amphotericin in the treatment of mucormycosis.
Since amphotericin is insoluble in an aqueous solution at physiological pH, the plain amphotericin B is combined with sodium deoxycholate to form a colloidal suspension. Amphotericin binds to ergosterol and results in pore formation. Pore formation leads to leakage of ions and, subsequently, cell death. It leads to potassium ion leakage in low doses, while high doses lead to magnesium ion leaks and, consequently, fungal cell death. Since amphotericin can bind to cholesterol in the human cell membrane, it can result in ion leakage resulting in hypokalaemia and hypomagnesemia. In this study, 68% of the patients developed hypokalaemia, while 47% developed hypomagnesemia. Despite the routine premedication protocol with 20 MEq of Potassium chloride every day, there was a high incidence of hypokalaemia. Although the development of dyselectrolytaemia is inevitable with amphotericin B, they are easily managed with routine monitoring and timely correction [15]. None of our patients required discontinuations due to electrolyte disturbances.
Amphotericin causes a dose-dependent constriction of afferent arterioles leading to decreased renal blood flow. This consequently leads to a reduced glomerular filtration rate. It is also directly toxic to distal renal tubules. In this study, 60% of the patients developed AKI on amphotericin treatment. Previous cohort studies have shown a 30-50% incidence of AKI on amphotericin treatment [16, 17]. The dose and duration of amphotericin can explain the variation in incidence. With increasing dose and duration, the incidence of AKI increased. In a study by Bicanic et al., creatinine increases by 52% on day 7 while it rose by 73% on day 14 [18]. In our study, the median day of development of AKI was 6 (4-10) days. In those patients who developed AKI, the salt loading was increased, and the infusion duration was prolonged. Although there is conflicting evidence about the benefits of prolonging infusion, increasing the salt loading decreased the incidence of AKI [19]. In a randomised controlled trial, nephrotoxicity was higher when 5% dextrose was used for premedication hydration than normal saline [15]. Studies show that nephrotoxicity due to amphotericin is reversible primarily [20]. However, resolution of creatinine can take months after cessation of amphotericin therapy [21]. The median increase from baseline to the last follow-up in creatinine was just 25% in our study. In most patients in our study, the creatinine at the last follow-up was less than 1.5 times the baseline. The therapy was not discontinued in any of the patients due to AKI in our series.
In a double-blind, randomised controlled trial that compared deoxycholate vs liposomal amphotericin for the management of cryptococcal meningitis, no difference in efficacy was noted [22]. Unfortunately, similar trials have not been conducted for mucormycosis. In an observational study from Mexico, the cure rate with deoxycholate amphotericin and surgical debridement was 55% in patients with mucormycosis [23]. In our study, only 14% of the patients succumbed to the illness during the median hospital stay of 17 days. In a systematic review by Watanabe et al., the pooled mortality of COVID-19-associated mucormycosis was 29% [24]. In our study, the rest of the patients who were doing well at discharge, 86% were prescribed posaconazole. The rest of the patients were not prescribed posaconazole due to financial constraints.
In resource-limited settings, patients with confirmed mucormycosis can be treated with deoxycholate amphotericin at a 1mg/kg dose. Potassium and creatinine can be monitored on alternate days initially. The frequency can be increased or decreased based on the condition of the patient. In those who develop hypokalaemia, magnesium levels can also be measured. It is a good practice to prophylactically supplement potassium before amphotericin B. In our centre, patients were given 500 mL to 1 litre of normal saline with 20 milliequivalents of potassium chloride prior to amphotericin. As discussed before, premedicating with normal saline can decrease AKI. In those who develop AKI, increasing hydration and decreasing infusion speed of amphotericin can be tried. Although there are no studies on appropriate duration, the usual protocol at our centre is to treat for at least 3-6 weeks, depending on the extent of involvement. These patients can then be transitioned to oral posaconazole.
Limitations: Due to the shorter follow-up duration, radiological improvement was not ascertained during follow-up. Also, since there was no comparison group, it isn’t easy to determine whether deoxycholate amphotericin has a similar impact on outcomes as liposomal amphotericin B.
In conclusion, considering the high mortality of mucormycosis in the absence of medical therapy, deoxycholate amphotericin is an acceptable and cheap alternative for treating mucormycosis in resource-constrained settings. Dyselectrolytaemia and AKI due to this drug is reversible and can be managed easily in such a setting.
REFERENCES
[1] Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India. Microorganisms. 2021; 9 (3), 523.
[2] Stone N, Gupta N, Schwartz I. Mucormycosis: time to address this deadly fungal infection. Lancet Microbe. 2021; 2 (8), e343-344.
[3] Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019; 19 (12), e405-421.
[4] Gupta N, Singh G, Xess I, Soneja M. Managing mucormycosis in a resource-limited setting: challenges and possible solutions. Trop Doct. 2019; 49(2), 153-155.
[5] Levey AS. Defining AKD: The Spectrum of AKI, AKD, and CKD. Nephron. 2022; 146(3), 302-305.
[6] Wasiq M, K R, Gn A. Coronavirus disease-associated mucormycosis (CAM): A case control study during the outbreak in India. J Assoc Physicians India. 2022; 70 (4), 11-12.
[7] Patel R, Jethva J, Bhagat PR, Prajapati V, Thakkar H, Prajapati K. Rhino-orbital-cerebral mucormycosis: An epidemiological study from a tertiary care referral center in Western India. Indian J Ophthalmol. 2022; 70 (4), 1371-1375.
[8] Sen M, Honavar SG, Bansal R, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021; 69 (7), 1670-1692.
[9] Morales-Franco B, Nava-Villalba M, Medina-Guerrero EO, et al. Host-Pathogen molecular factors contribute to the pathogenesis of Rhizopus spp. in diabetes mellitus. Curr Trop Med Rep. 2021; 1-12.
[10] Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg Infect Dis. 2021; 27(9), 2349-2359.
[11] Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022 Jan 25;
[12] Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008; 47(4), 503-509.
[13] Soman R, Chakraborty S, Joe G. Posaconazole or isavuconazole as sole or predominant antifungal therapy for COVID-19-associated mucormycosis. A retrospective observational case series. Int J Infect Dis. 2022; 120, 177-178.
[14] Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016; 16 (7), 828-837.
[15] Llanos A, Cieza J, Bernardo J, et al. Effect of salt supplementation on amphotericin B nephrotoxicity. Kidney Int. 1991; 40 (2), 302-308.
[16] Wingard JR, Kubilis P, Lee L, et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis. 1999; 29 (6), 1402-1407.
[17] Bates DW, Su L, Yu DT, et al. mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001; 32 (5), 686-693.
[18] Bicanic T, Bottomley C, Loyse A, et al. Toxicity of Amphotericin B Deoxycholate-Based Induction Therapy in patients with HIV-Associated Cryptococcal Meningitis. Antimicrob Agents Chemother. 2015; 59 (12), 7224-7231.
[19] Anderson CM. Sodium chloride treatment of amphotericin B nephrotoxicity. Standard of care? West J Med. 1995; 162 (4), 313-317.
[20] Medoff G, Kobayashi GS. Strategies in the treatment of systemic fungal infections. N Engl J Med. 1980; 302 (3), 145-155.
[21] Butler WT, Bennett JE, Alling DW, Wertlake PT, Utz JP, Hill GJ. Nephrotoxicity of Amphotericin B; early and late effects in 81 patients. Ann Intern Med. 1964; 61, 175-187.
[22] Hamill RJ, Sobel JD, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomised, double-blind clinical trial of efficacy and safety. Clin Infect Dis. 2010; 51 (2), 225-232.
[23] Bonifaz A, Tirado-Sánchez A, Hernández-Medel ML, et al. Mucormycosis at a tertiary-care center in Mexico. A 35-year retrospective study of 214 cases. Mycoses. 2021; 64 (4), 372-380.
[24] Watanabe A, So M, Mitaka H, et al. Clinical features and mortality of COVID-19-Associated Mucormycosis: a systematic review and meta-analysis. Mycopathologia. 2022 Mar 21.