Le Infezioni in Medicina, n. 3, 427-431, 2022
doi: 10.53854/liim-3003-11
ORIGINAL ARTICLES
Are SARS-CoV-2 rapid antigen tests useful for the control of latest variants spreading?
Nadia Marascio1, Angela Quirino1, Giuseppe Guido Maria Scarlata1, Giorgio Settimo Barreca1, Aida Giancotti1, Angelo Giuseppe Lamberti1, Luigia Gallo1, Fabio Foti1, Domenico Luca Laurendi2, Daniela Dattola3, Antonino Marsico4, Antonia La Rocca4, Giovanni Matera1
1Department of Health Sciences, Unit of Microbiology, “Magna Graecia” University, Catanzaro, Italy;
2Italian Association of Biologists, Reggio Calabria, Italy;
3Italian Red Cross, Reggio Calabria Committee, Italy;
4Diocesan Caritas, Reggio Calabria-Bova, Italy
Article received 25 July 2022, accepted 5 August 2022
Corresponding author
Angela Quirino
E-mail: quirino@unicz.it
SummaRY
Reverse Transcription Polymerase Chain Reaction (RT-PCR) conducted on nasopharyngeal swabs is the gold standard in the diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). In Italy, recent guidelines indicate that rapid antigen tests (RATs) can be used for the isolation of positive patients or for the interruption of quarantine, but they are often less sensitive to detect positive subjects. Indeed, the performance of these RATs depends on the timing and the population on which they are evaluated. Herein, we evaluated the performance of BIOCREDIT COVID-19 Ag and Fluorecare® SARS-CoV-2 Spike Protein Test during a population screening in the Calabria Region, Southern Italy. We report that both antigen test shows low sensitivity in contrast to the high sensitivity declared by manufacturer (90% and 92%, respectively) and that the area under the curve (AUC) was good for Fluorecare® SARS-CoV-2 Spike Protein Test but very poor for BIOCREDIT COVID-19 Ag. We suggest that these RATs should be re-evaluated in the current pandemic era.
Keywords: SARS-CoV-2, Rapid antigen tests; RT-PCR.
INTRODUCTION
The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified as a pathogen of atypical pneumonia in Wuhan, China in January 2020 [1]. SARS-CoV-2 single-stranded RNA genome, encodes nine accessory proteins and four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) [2]. Specifically, the S protein is involved in the viral entry, while N protein packages the viral genome into a long helical ribonucleocapsid (RNP) complex and participates in the assembly of the virion [3]. Actually, Reverse Transcription Polymerase Chain Reaction (RT-PCR) conducted on nasopharyngeal swabs is the diagnostic gold standard, due to its high sensitivity and specificity (98% and 97%, respectively), but at the same time, it is a highly expensive test requiring highly qualified staff [4]. Due to the heavy pressure imposed by the current pandemic on the diagnostic routine, rapid antigen tests (RATs) provide for the detection of S or N antigen on nasopharyngeal swabs with significantly lower costs, obtaining results in a shorter time (about 15 minutes) than RT-PCR [5]. However, the high number of deletions or substitutions in the S (such as E484K and K417N/T) and N (such as R203K and G204R) proteins, that allow SARS-CoV-2 to elude the host immune system, could impair the performance of these RATs, increasing the number of false negatives [6]. In Italy, recent guidelines indicate that RATs can be used for the isolation of positive patients or for the interruption of quarantine [7]. Currently, the World Health Organization (WHO) recommends the use of highly sensitive and specific RATs (80% and 97%, respectively) to monitor early stages of epidemic outbreaks [8,9]. However, it is known that the performance of these RATs depends on the timing and the population on which they are evaluated. The aim of the study was to evaluate the performance of two RATs during a population screening in the Calabria Region, Southern Italy.
PATIENTS AND METHODS
Ethical statement
Clinical data collection was performed in accordance with the principles of the Helsinki Declaration (64th WMA General Assembly, Fortaleza, Brazil, October 2013). Written informed consent was obtained from volunteers subjects for this study.
Patients and samples collection
In January 2021, a total of 159 consecutive subjects were enrolled in one day. Subjects of all age groups who were asymptomatic or pauci-symptomatic were included. Confirmed COVID-19 cases by gold standard RT-PCR were excluded. The screened people provided demographic characteristics, symptoms and reason for testing. We carried out on each of the enrolled subjects three nasopharyngeal swabs. Two of these were immediately processed using RATs, while the remaining nasopharyngeal swab to be subsequently analyzed by RT-PCR were placed in Universal Transport Medium® for viruses (UTM) and stored at - 80°C.
BIOCREDIT COVID-19 Ag (RapiGEN INC, Anyang, Korea)
It is a lateral flow immunoassay using a dual-color system. This test contains a colloidal gold buffer conjugated with a membranous strip pre-coated with antibodies specific for the SARS-CoV-2 N antigen on the test line (T). If SARS-CoV-2 N antigen is present in the sample a visible black band appears on the test lines indicating the formation of the gold-conjugated antigen-antibody complex. The control line (C) is used to control the procedure and appears if the test has been performed correctly. The presence of a single red line on the control band indicates a negative result, whereas if a red line appears on the control band and a black line on the test band the result is considered positive. The result is invalid if the red stripe on the control band does not appear and therefore the test must be repeated. Sensitivity and specificity, declared by manufacturer and evaluated on 187 SARS-CoV-2 positive patients by RT-PCR, are 90.2% and 100%, respectively.
Fluorecare® SARS-CoV-2 Spike Protein Test (Microprofit Biotech, Shenzhen, China)
It is an immunochromatography assay used to determine the presence of the SARS-CoV-2 Spike protein which, if present, upon binding to the fluorescently labelled anti-SARS-CoV-2 antibody will form an immune complex evidenced by specific control (C) and test (T) bands. The dedicated analyzer (fluorecare® MF-T1000) can read the results after the reaction is complete. The SARS-CoV-2 spike protein concentration was calculated using a predefined calibration curve in accordance with the manufacturer’s instructions. Sensitivity and specificity, declared by manufacturer and evaluated on 351 SARS-CoV-2 positive patients by RT-PCR, are 92.16% and 100%, respectively.
Allplex™ SARS-CoV-2 Assay Kit (Seegene, Republic of Korea)
It is a Real-Time Multiplex PCR that detects RdRp/S and N genes specific for SARS-CoV-2, and the E gene for all Sarbecovirus including SARS-CoV-2. The test is highly sensitive, specific and accurate with a Limit of Detection (LoD) of 50 copies per reaction and has no cross-reactivity with 54 respiratory pathogens including SARS-CoV and MERS-CoV. The results were interpreted by specific Data Analysis software in accordance with the manufacturer’s instructions. The Cycle threshold (Ct) value was related to SARS-CoV-2 RNA viral load as following: Ct<25 = high, 25<Ct <30 = intermediate, Ct>30 = low.
Statistical analysis
Statistics was carried out by ROC analysis using GraphPad Prism v8.4.3.
RESULTS
Among 159 enrolled people, 7/159 (4.4%) were RT-PCR positive by Allplex™ SARS-CoV-2 Assay Kit, the mean Ct values were 23, 25, 33, 35, 36, 37 and 39, respectively. Overall, 69 (43.4%) were males and 90 (56.6%) females. Median age was 44 (range 8 - 89) years old. Two of the enrolled subjects claimed to have symptoms, while ten of enrolled subjects referred contacts with positive subjects. None of them resulted positive to the three tests. Only two subjects who showed mean Ct values < 30 were positive for the two antigen tests or one test (Fluorecare® test), respectively. BIOCREDIT COVID-19 Ag identified two positive subjects, but only one was confirmed by RT-PCR (mean Ct value 25). Fluorecare® SARS-CoV-2 Spike Protein Test, detected ten positive subjects, but only three were confirmed by RT-PCR (mean Ct values were 23; 25; 39, respectively), as show in Table 1. A total of 145 swabs were negative to all methods reported in our study. Statistical analysis displayed lower sensitivity than declared by manufacturer (90% for BIOCREDIT COVID-19 Ag and 92% for Fluorecare® SARS-CoV-2 Spike Protein Test, respectively) and a high specificity in accordance with declared by the manufacturer (100% for both RATs). BIOCREDIT COVID-19 Ag (targeting N protein) showed a sensitivity of 14% and a specificity of 99% with AUC of 0.568, while Fluorecare® SARS-CoV-2 Spike Protein Test showed a sensitivity of 42% and a specificity of 95% with AUC of 0.690 (Figure 1).

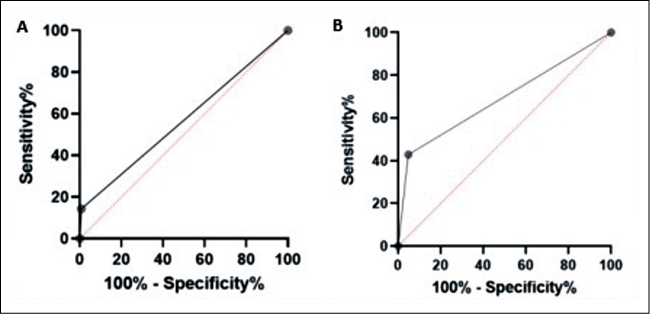
Figure 1 - ROC analysis of BIOCREDIT COVID-19 Ag (Panel A) and Fluorecare® SARS-CoV-2 Spike Protein Test (Panel B).
DISCUSSION
Our study report that both antigen test shows low sensitivity in contrast to the high sensitivity declared by manufacturer (90% and 92%, respectively). However, the area under the curve (AUC=0.690) in our study is acceptable for Fluorecare® SARS-CoV-2 Spike Protein Test, while it is very poor for the other antigen test evaluated. In the literature, these tests have been evaluated mainly on confirmed COVID-19 cases or symptomatic subjects. On the contrary, the present investigation was carried out on basically asymptomatic patients. Regarding BIOCREDIT COVID-19 Ag, one of the first studies compared the performance of this RAT to RT-PCR and viral cultures, showing that it detected only 11-45% of true positives [10]. A more recent study, performed on symptomatic and asymptomatic patients from isolation centers and on patients entered in Uganda, showed 59% accuracy, but low sensitivity in asymptomatic or low viral load patients (21% and 27%, respectively) [11]. A study, carried out on 119 symptomatic patients, showed BIOCREDIT COVID-19 sensitivity of 8%, advising against its use even beside RT-PCR [12]. Regarding Fluorecare® SARS-CoV-2 Spike Protein Test, Tonelotto and colleagues evaluated it in population screening on 253 nasopharyngeal swabs, showing high sensitivity and specificity (84.6% and 100%, respectively). Therefore, the authors suggest the introduction of this test into routine diagnostics [13]. On the other hand, Salvagno and co-workers reported a modest sensitivity (27%), but a good specificity (99%) in a screening performed on 354 hospitalized patients [14]. Sensitivity of these two antigen tests is lower than other Food and Drug Administration (FDA) approved RATs, such as the BinaxNOWTM COVID-19 Ag Card Home Test (Abbott Diagnostics Scarborough, Inc, USA) (84%), CareStart COVID-19 Antigen Home Test (Access Bio, Inc, New Jersey, USA) (87.2%) and Celltrion DiaTrust COVID-19 Ag Rapid Test (Celltrion Inc, USA) (93%) [15]. Additionally, recent rapid microfluidic immunofluorescence point-of-care antigen test, such as LumiraDx SARS-CoV-2 Ag Test (LumiraDX, UK), have demonstrated good accuracy with results comparable to RT-PCR, suggesting their use for rapid screening of asymptomatic individuals in both community and hospital settings [16] Limitations of this study includes the small number of patients enrolled, which made it impossible to assess the positive predictive value (PPV) and negative predictive value (NPV) of the two RATs, and the impossibility of enrolling new volunteers subjects for a population screening in the current epidemiological scenario. During B.1 and B.1.1.7 variants circulation, these rapid antigen tests were able to detect, in the majority of cases, positive subjects with high or intermediate SARS-CoV-2 RNA viral load. Fluorecare® and BIOCREDIT tests should be re-evaluated in the current pandemic era, taking into account viral load levels and the emergence of new SARS-CoV-2 Omicron (BA.1.1.529) variant and their sub-lineages (such as BA.2, BA.4 and BA.5) bearing major mutations in the S and N proteins.
Conflict interest
All authors declared no conflict of interest.
Funding
This manuscript didn’t receive external funding.
References
[1] Zhu N., Zhang D., Wang W., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020; 382 (8), 727-733.
[2] Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009; 7 (6), 439-450.
[3] Bai Z., Cao Y., Liu W., Li J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses. 2021; 13 (6), 1115.
[4] Oliveira B.A., Oliveira L.C., Sabino E.C., Okay T.S. SARS-CoV-2 and the COVID-19 disease: a mini review on diagnostic methods. Rev Inst Med Trop Sao Paulo. 2020; 62, e44.
[5] Jeewandara C., Guruge D., Pushpakumara P.D., et al. Sensitivity and specificity of two WHO approved SARS-CoV2 antigen assays in detecting patients with SARS-CoV2 infection. BMC Infect Dis. 2022; 22 (1), 276.
[6] Thakur S., Ssi S., Pillai S.G., et al. SARS-CoV-2 Mutations and Their Impact on Diagnostics, Therapeutics and Vaccines. Front Med (Lausanne). 2022; 9, 815389.
[7] Ministero della Salute (2021), Aggiornamento sull’uso dei test antigenici e molecolari per la rilevazione di SARS-CoV-2. https://www.quotidianosanita.it/allegati/allegato2672387.pdf
[8] Centre for Disease Control U. Interim guidance for antigen testing for SARS-CoV-2. In: National center for immunization and respiratory diseases (NCIRD), Division of Viral Diseases; 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html
[9] Peeling R.W., Olliaro P.L., Boeras D.I., Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021; 21 (9), e290-5.
[10] Mak G.C., Cheng P.K., Lau S.S., et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020; 129, 104500.
[11] Bwogi J., Lutalo T., Tushabe P., et al. Field evaluation of the performance of seven Antigen Rapid diagnostic tests for the diagnosis of SARs-CoV-2 virus infection in Uganda. PLoS One. 2022; 17 (5), e0265334.
[12] Kenyeres B., Ánosi N., Bányai K., et al. Comparison of four PCR and two point of care assays used in the laboratory detection of SARS-CoV-2. J Virol Methods. 2021; 93, 114165.
[13] Tonelotto V., Davini A., Cardarelli L., Calderone M., Marin P. Efficacy of Fluorecare SARS-CoV-2 Spike Protein Test Kit for SARS-CoV-2 detection in nasopharyngeal samples of 121 individuals working in a manufacturing company. PLoS One. 2022; 17 (1), e0262174.
[14] Salvagno G.L., Gianfilippi G., Pighi L., et al. Real-world assessment of Fluorecare SARS-CoV-2 Spike Protein Test Kit. Adv Lab Med. 2021; 2 (3), 409-412.
[15] In Vitro Diagnostics EUAs - Antigen Diagnostic Tests for SARS-CoV-2, 2022. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas
[16] Drain P., Sulaiman R., Hoppers M., Lindner N.M., Lawson V., Ellis J.E. Performance of the LumiraDx Microfluidic Immunofluorescence Point-of-Care SARS-CoV-2 Antigen Test in Asymptomatic Adults and Children. Am J Clin Pathol. 2022; 157 (4), 602-607.